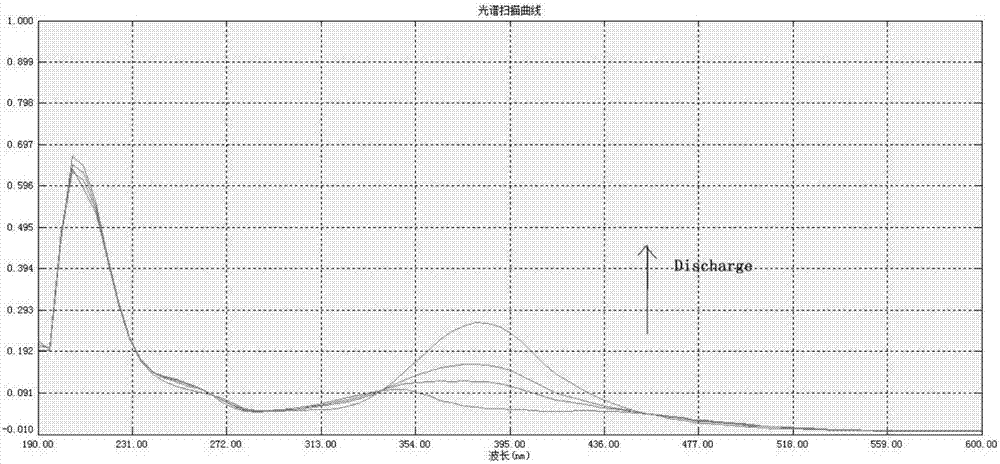
Heterocyclic azobenzene high-polymer energy storage material and preparation method thereof
A technology of heterocyclic azobenzene and energy storage materials, applied in the direction of heat exchange materials, chemical instruments and methods, etc., can solve the problems of energy storage limitations, low energy density of azobenzene, short half-life, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
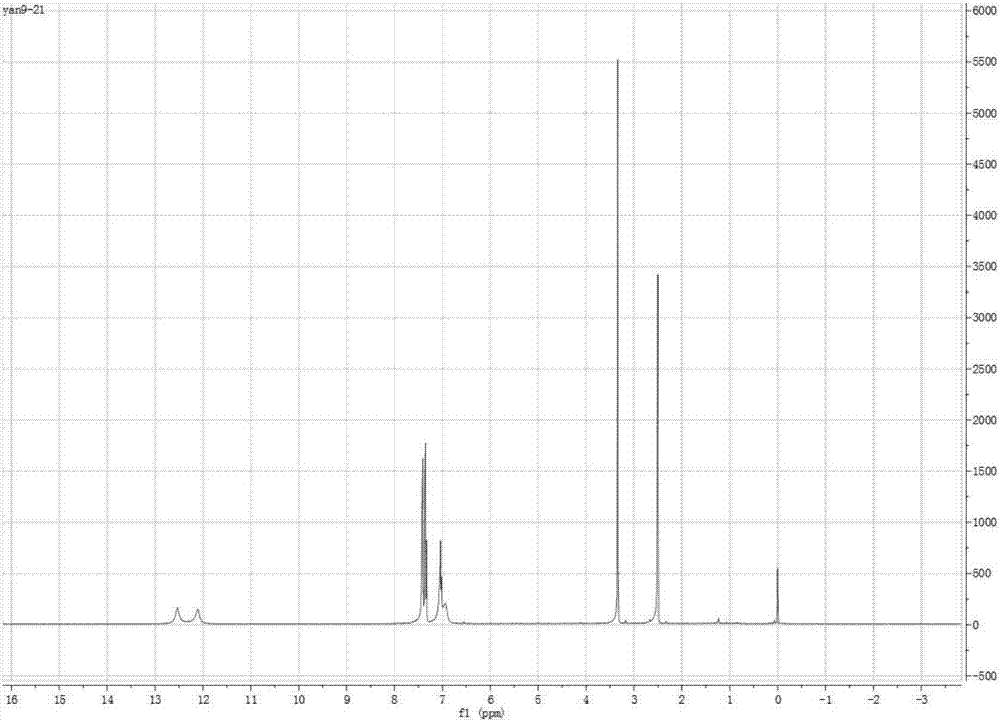
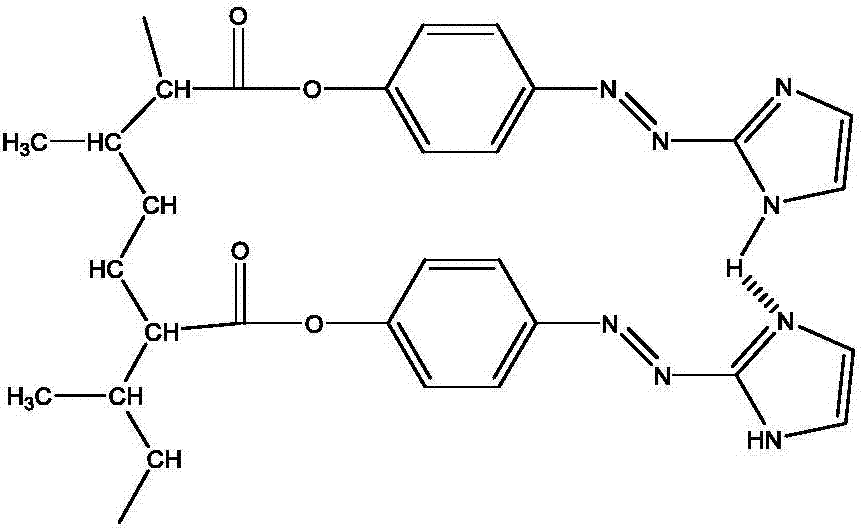
[0029] (1) Preparation of azo monomer: 0.03 mol of 2-aminoimidazole was dissolved in a mixture of 1 mol / L hydrochloric acid, ice and acetone. An aqueous solution containing 30 ml of 0.03 mol / L sodium nitrite was slowly added to the above mixture. The mixture was stirred under ice bath for 20 minutes. Then, an aqueous solution of 0.03 mol of phenol in 1 mol / L sodium hydroxide was added to the mixture. The reaction mixture was stirred at pH 8 for 3 hours. After neutralizing with 1 mol / L HCl, the resulting mixture was filtered and washed with water. The crude product was dried and purified by recrystallization from ethanol to afford the compound as a reddish-brown solid.
[0030] (2) Preparation of alkenyl azo monomer: under ice-water bath conditions, 0.01mol of methacryloyl chloride was dissolved in a certain amount of anhydrous CH 2 Cl 2 The solution in is added dropwise to the solution containing 0.01mol p-hydroxyazoimidazole, 0.01mol triethylamine and anhydrous CH 2 Cl ...
Embodiment 2
[0033] (1) Preparation of azo monomer: 0.06 mol of 2-aminoimidazole was dissolved in a mixture of 1 mol / L hydrochloric acid, ice and acetone. An aqueous solution containing 30 ml of 0.03 mol / L sodium nitrite was slowly added to the above mixture. The mixture was stirred under ice bath for 20 minutes. Then, an aqueous solution of 0.03 mol of phenol in 1 mol / L sodium hydroxide was added to the mixture. The reaction mixture was stirred at pH 8 for 3 hours. After neutralizing with 1 mol / L HCl, the resulting mixture was filtered and washed with water. The crude product was dried and purified by recrystallization from ethanol to afford the compound as a reddish-brown solid.
[0034] (2) Preparation of alkenyl azo monomer: under ice-water bath conditions, 0.01mol of methacryloyl chloride was dissolved in a certain amount of anhydrous CH 2 Cl 2 The solution in is added dropwise to the solution containing 0.01mol p-hydroxyazoimidazole, 0.01mol triethylamine and anhydrous CH 2 Cl ...
Embodiment 3
[0037] (1) Preparation of azo monomer: 0.1 mol of 2-aminoimidazole was dissolved in a mixture of 1 mol / L hydrochloric acid, ice and acetone. An aqueous solution containing 30 ml of 0.03 mol / L sodium nitrite was slowly added to the above mixture. The mixture was stirred under ice bath for 20 minutes. Then, an aqueous solution of 0.03 mol of phenol in 1 mol / L sodium hydroxide was added to the mixture. The reaction mixture was stirred at pH 8 for 3 hours. After neutralizing with 1 mol / L HCl, the resulting mixture was filtered and washed with water. The crude product was dried and purified by recrystallization from ethanol to afford the compound as a reddish-brown solid.
[0038] (2) Preparation of alkenyl azo monomer: under ice-water bath conditions, 0.01mol of methacryloyl chloride was dissolved in a certain amount of anhydrous CH 2 Cl 2 The solution in is added dropwise to the solution containing 0.01mol p-hydroxyazoimidazole, 0.01mol triethylamine and anhydrous CH 2 Cl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com