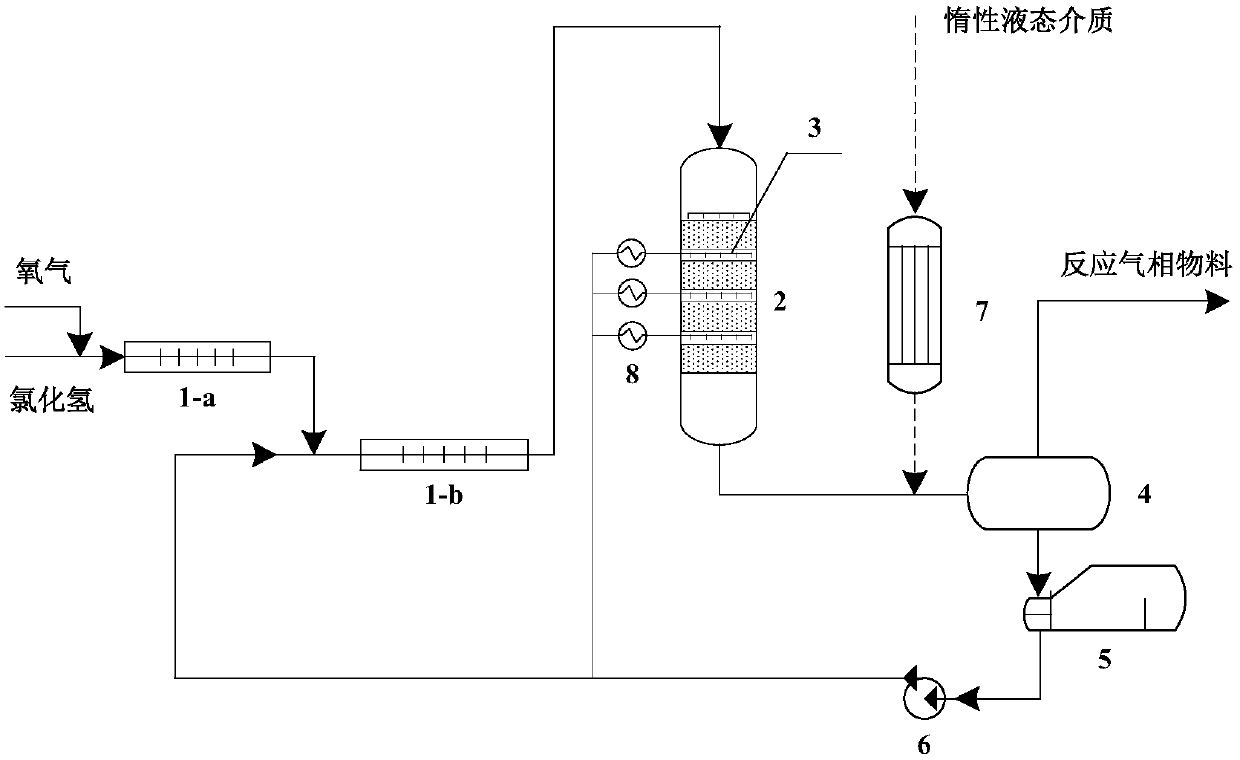
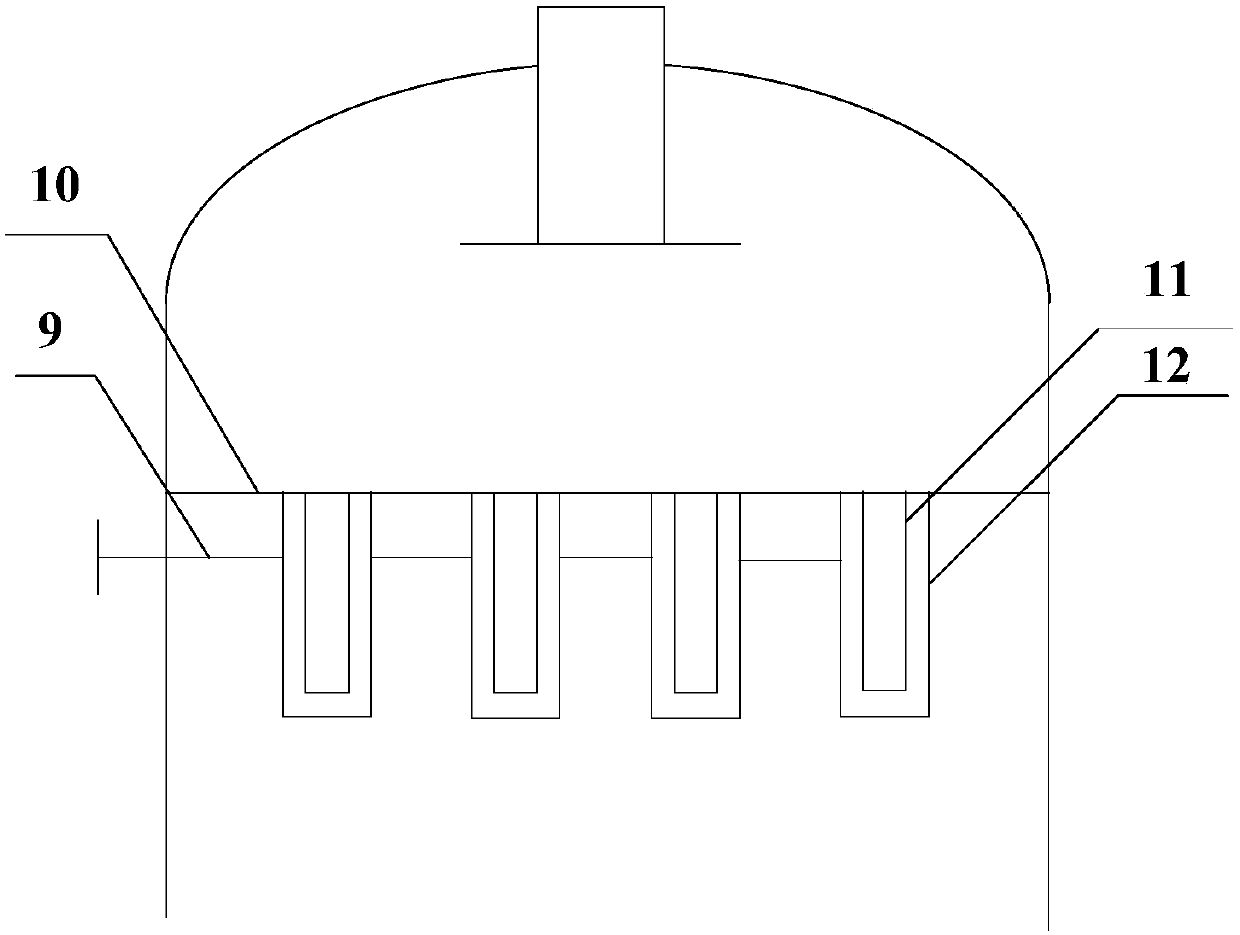
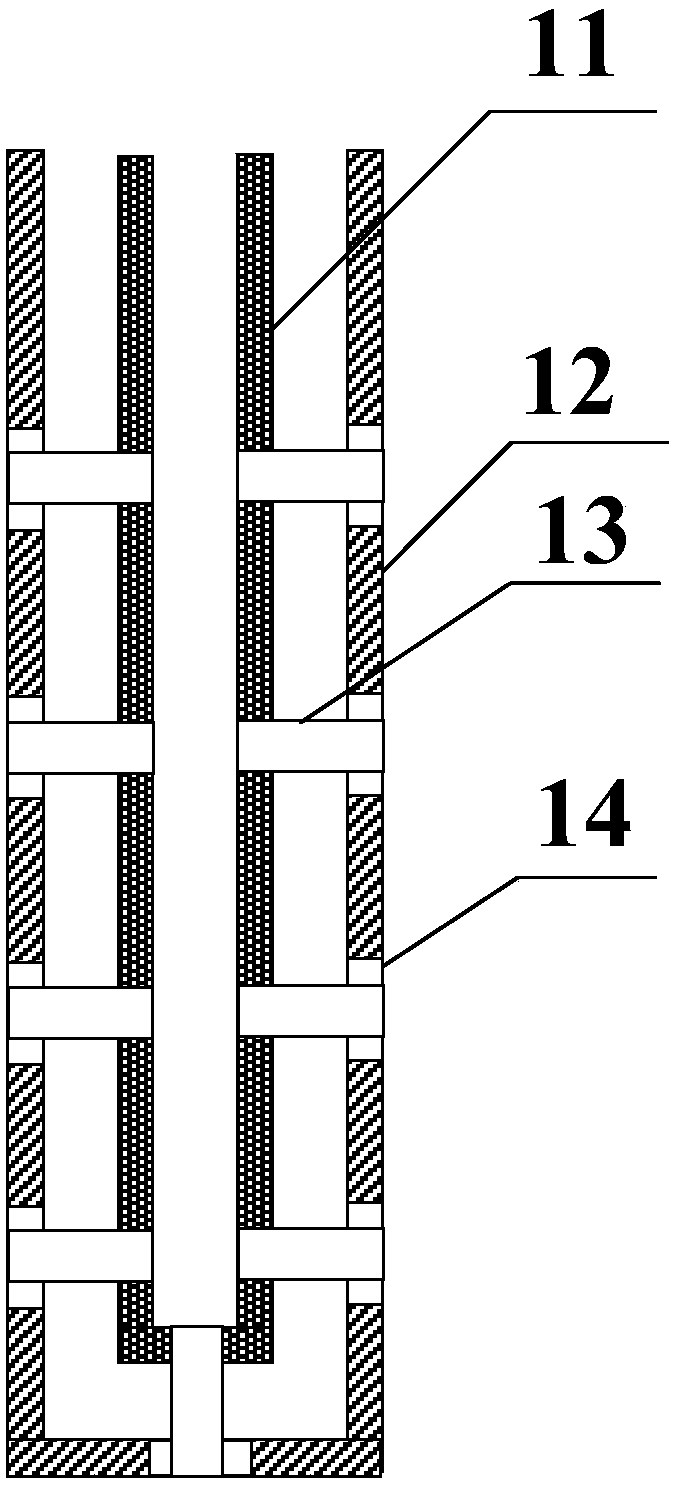
A kind of method that adopts trickle-bed reactor to carry out hydrogen chloride oxidation to prepare chlorine
A reactor and trickle bed technology, applied in the field of hydrogen chloride oxidation to prepare chlorine, can solve the problems of poor industrial adaptability, difficult temperature control, low conversion rate of hydrogen chloride, etc., and achieve less side reactions in the liquid phase, avoid recovery, and high conversion rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] Preparation of Catalyst 1
[0117] will contain 30g CuCl 22H 2 O, and containing dispersant 0.5g potassium oxalate cuprate dihydrate (K 2 [Cu(C 2 o 4 ) 2 ]·2H 2 O) in 50ml of aqueous solution, impregnated in 50g blank carrier γ-alumina powder for more than 12hr, then dried at 90°C for 16hr, and redispersed the obtained solid in a solution containing 1.2gKCl and 8g Ce(NO 3 ) 3 ·Immerse again in 100ml of 6H2O aqueous solution for more than 12 hours, and then dry at 90°C for 16 hours. The catalyst was extruded and then calcined at 500° C. for 4 hours to obtain a solid active catalyst 1 . Finally, the atomic ratio of copper, potassium and cerium in the catalyst is about 1:0.1:0.1, and the total amount of metal salt and oxide in the catalyst is 21.02g.
Embodiment 2
[0119] Preparation of Catalyst 2
[0120] will contain 4.7g CuCl 2 2H 2 O. Potassium oxalate cuprate dihydrate containing 0.5 g of dispersant (K 2 [Cu(C 2 o 4 ) 2 ]·2H 2 O) in 50ml of aqueous solution, impregnated with 50g blank carrier HY molecular sieve for more than 12hr, then dried at 90°C for 16hr, and redispersed the obtained solid in a solution containing 12.5g Ce(NO 3 ) 3 ·6H 2 In 100ml of aqueous solution of O, soak again for more than 12 hours, and then dry at 90°C for 16 hours. The catalyst was extruded and then calcined at 500°C for 4 hours to obtain catalyst 2. Finally, the atomic ratio of copper, potassium and cerium in the catalyst is about 1:0.1:1, and the final total of metal salt and oxide in the catalyst is 7.22g.
Embodiment 3
[0122] Preparation of Catalyst 3
[0123] will contain 8g CuCl 2 2H 2 O. Potassium oxalate cuprate dihydrate containing 2.5 g of dispersant (K 2 [Cu(C 2 o 4 ) 2 ]·2H 2 O) in 50ml of aqueous solution, impregnated with 50g of blank carrier HY molecular sieve for more than 12hr, then dried at 90°C for 16hr, and redispersed the obtained solid in a solution containing 11.2g of Ce(NO 3 ) 3 ·6H 2 O. Immerse again in 100ml of 3.5g KCl aqueous solution for more than 12hr, and then dry at 90°C for 16hr. The catalyst was extruded and then calcined at 500° C. for 4 hours to obtain catalyst 3 . Finally, the atomic ratio of copper, potassium and cerium in the catalyst is about 1:0.75:0.5, and the final total of metal salt and oxide in the catalyst is 11.5g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com