Tetrazole cyano boric acid ionic liquid and preparation method thereof
A technology of tetrazolium cyanoboronic acid and ionic liquid, applied in chemical instruments and methods, offensive equipment, generation of compressed gas, etc., can solve problems such as unfavorable large-scale synthesis, cumbersome synthesis steps, gaps in density, energy and specific impulse, etc. , to achieve the effect of wide liquid range, safe operation and short ignition delay
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
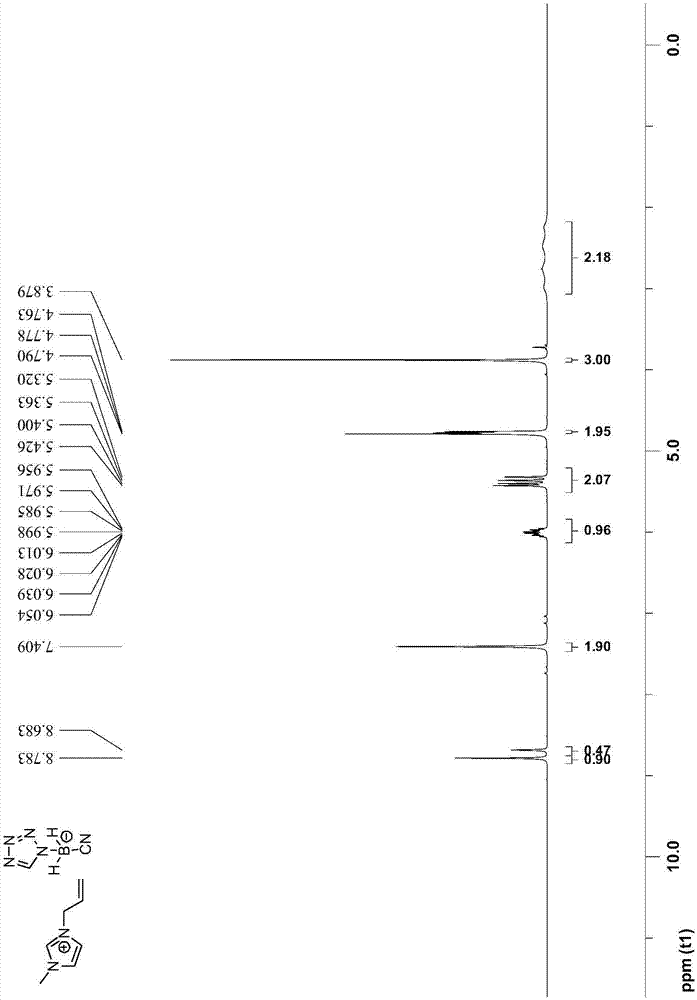
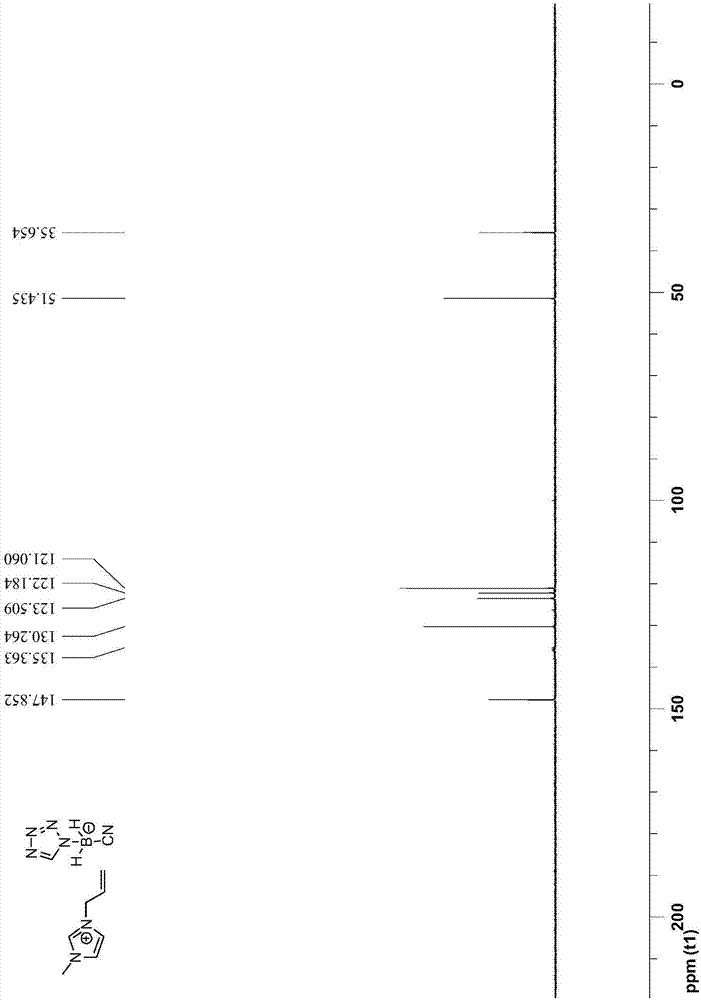
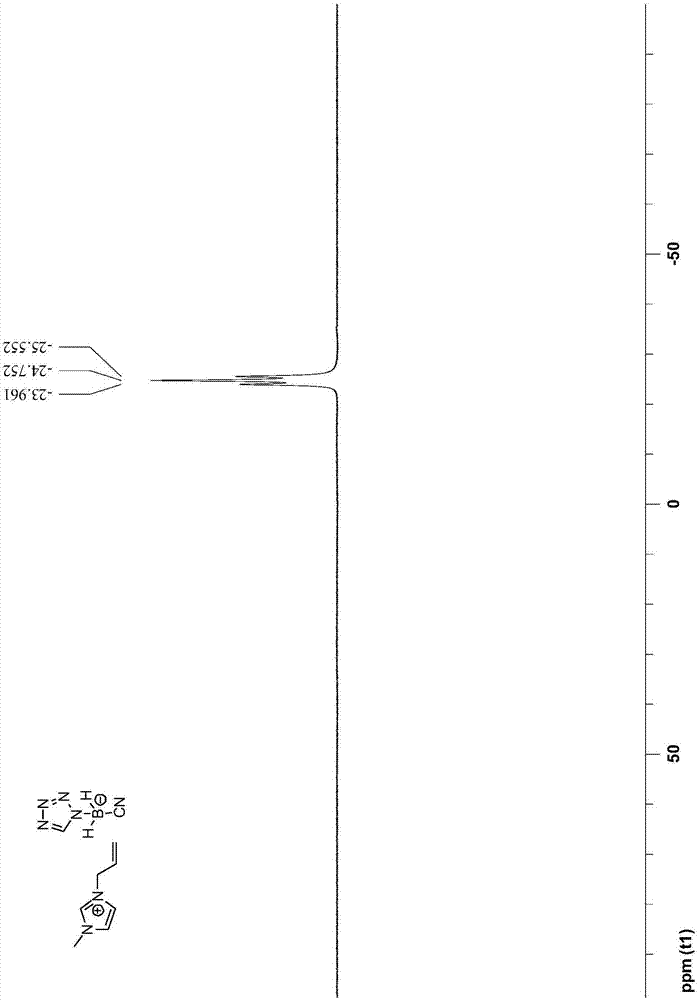
[0048] The preparation steps of 1-allyl-3-methyl-1H-imidazole tetrazolium cyanoborate are as follows:
[0049] (1) Under the protection of argon, first add 100mmol sodium cyanoborohydride to 70mL toluene, then add 100mmol 1H-tetrazole, then reflux and stir at 120°C for 6h, cool to room temperature, filter and collect the solid Substance; then mixed solution of tetrahydrofuran and dioxane (V 四氢呋喃 :V 二氧六环 =10:1) recrystallize the collected solid matter, filter to obtain the recrystallized solid, and vacuum-dry to obtain sodium tetrazolium cyanoborate;
[0050] (2) After adding 20mmol 1-allyl-3-methyl-1H-imidazolium chloride salt and 24mmol sodium tetrazolium cyanoborate into 30mL acetonitrile, stir the reaction at 25°C for 7 days, filter and collect the filtrate ; After the acetonitrile solvent in the filtrate was removed by rotary evaporation, dissolve with 50mL dichloromethane, then wash with water three times, then dry with anhydrous sodium sulfate, then carry out vacuum di...
Embodiment 2
[0060] Synthesis of 1-ethyl-3-methyl-1H-imidazolium tetrazolium cyanoborate
[0061] (1) Under the protection of argon, first add 100mmol sodium cyanoborohydride to 70mL toluene, then add 100mmol 1H-tetrazole, then reflux and stir at 120°C for 6h, cool to room temperature, filter and collect the solid Substance; then mixed solution of tetrahydrofuran and dioxane (V 四氢呋喃 :V 二氧六环 =10:1) recrystallize the collected solid matter, filter to obtain the recrystallized solid, and vacuum-dry to obtain sodium tetrazolium cyanoborate;
[0062] (2) Add 20mmol 1-ethyl-3-methyl-1H-imidazolium chloride salt and 24mmol sodium tetrazolium cyanoborate to 30mL acetonitrile, stir and react at 25°C for 7 days, filter and collect the filtrate; After the acetonitrile solvent in the filtrate was removed by rotary evaporation, it was first dissolved with 50 mL of dichloromethane, then washed with water three times, then dried with anhydrous sodium sulfate, then subjected to vacuum distillation, and ...
Embodiment 3
[0072] Synthesis of 1-ethyl-pyridine tetrazolium cyanoborate
[0073] (1) Under the protection of argon, first add 100mmol sodium cyanoborohydride to 70mL toluene, then add 100mmol 1H-tetrazole, then reflux and stir at 120°C for 6h, cool to room temperature, filter and collect the solid Substance; then mixed solution of tetrahydrofuran and dioxane (V 四氢呋喃 :V 二氧六环 =10:1) recrystallize the collected solid matter, filter to obtain the recrystallized solid, and vacuum-dry to obtain sodium tetrazolium cyanoborate;
[0074] (2) Add 20mmol 1-ethyl-pyridinium bromide and 24mmol sodium tetrazolium cyanoborate to 30mL of acetonitrile, stir and react at 25°C for 7 days, filter and collect the filtrate; remove the acetonitrile in the filtrate by rotary evaporation After the solvent was dissolved, it was first dissolved with 50 mL of dichloromethane, then washed with water three times, then dried with anhydrous sodium sulfate, then subjected to vacuum distillation, and finally vacuum-dri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Phase transition temperature | aaaaa | aaaaa |
| Decomposition temperature | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com