Cu-ZnO/SiO2 aerogel bimetallic catalyst, as well as preparation method and application thereof
A bimetallic catalyst and airgel technology, applied in the direction of physical/chemical process catalysts, chemical instruments and methods, and the preparation of organic compounds, can solve the problems of single raw materials, high price anhydrous methanol, and low equipment investment, and achieve The effect of simple preparation process, good catalytic activity and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
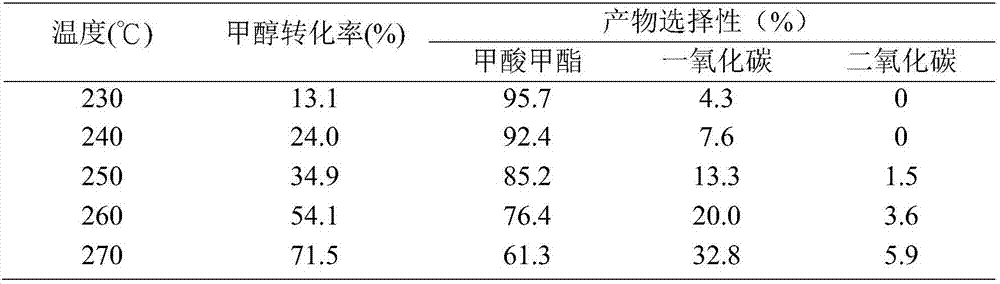
Embodiment 1
[0017] Catalyst preparation:
[0018] Cu-ZnO / SiO 2 Airgel (Cu:ZnO:SiO 2 Airgel = 10:2:88, W / W) The catalyst was prepared by co-precipitation method: Weigh 8.8g of SiO 2 Add the airgel into a three-neck flask containing 200ml of deionized water, and stir rapidly in a constant temperature water bath at 65°C to make it evenly dispersed. Prepare 1mol L -1 Na 2 CO 3 For 1L of solution, 3.8009g of Cu(NO 3 ) 2 ·3H 2 O and 0.7311g of Zn(NO 3 ) 2 ·6H 2 O mixed aqueous solution and Na 2 CO 3 The solution was added dropwise into a three-necked flask, stirred vigorously, and adjusted by Na 2 CO 3 The flow velocity of solution makes the pH value of the reaction mixture in the flask stabilize between 7.5 ± 0.2, when Cu(NO 3 ) 2 ·3H 2 O and Zn(NO 3 ) 2 ·6H 2 After the O mixed aqueous solution was added completely, the stirring was continued for 2h. Then filter and wash with deionized water. After washing, the sample was dried overnight at 120° C., calcined at 450° C. for...
Embodiment 2
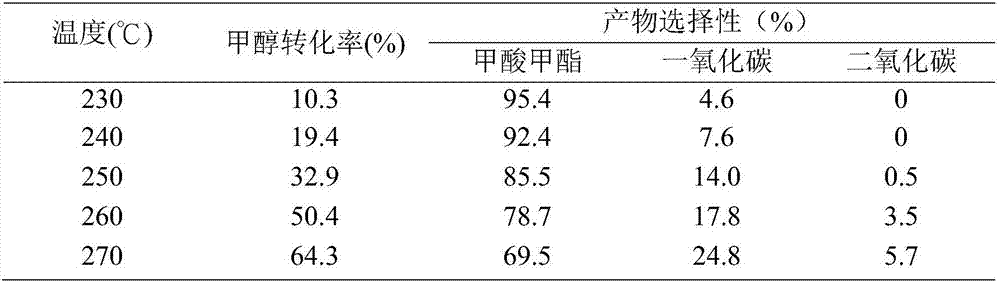
[0026] Same as Example 1, but changing the loadings of Cu and ZnO in the catalyst so that the loadings of Cu and ZnO are 10% and 3% respectively, the results are shown in Table 2.
[0027] Table 2. Cu(10)-ZnO(3) / SiO 2 Feedstock conversion and reaction product selection for airgel-catalyzed methanol dehydrogenation
[0028]
Embodiment 3
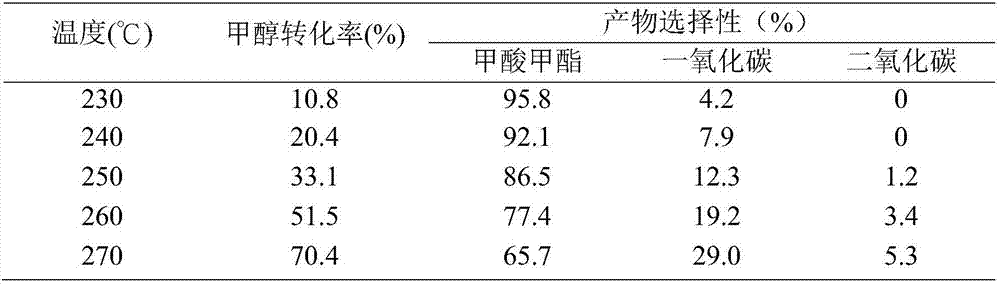
[0030] Same as Example 1, but changing the loading of Cu and ZnO in the catalyst, so that the loading of Cu and ZnO are 20% and 3% respectively, the results are shown in Table 3.
[0031] Table 3. Cu(20)-ZnO(3) / SiO 2 Feedstock conversion and reaction product selection for airgel-catalyzed methanol dehydrogenation
[0032]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com