Thermophilic neutral protease gene, engineering bacteria, enzyme and application thereof
A technology of neutral protease and gene, applied in the field of thermophilic neutral protease gene, engineering bacteria, enzyme and its application, can solve problems such as complex conditions, limited application range, poor stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
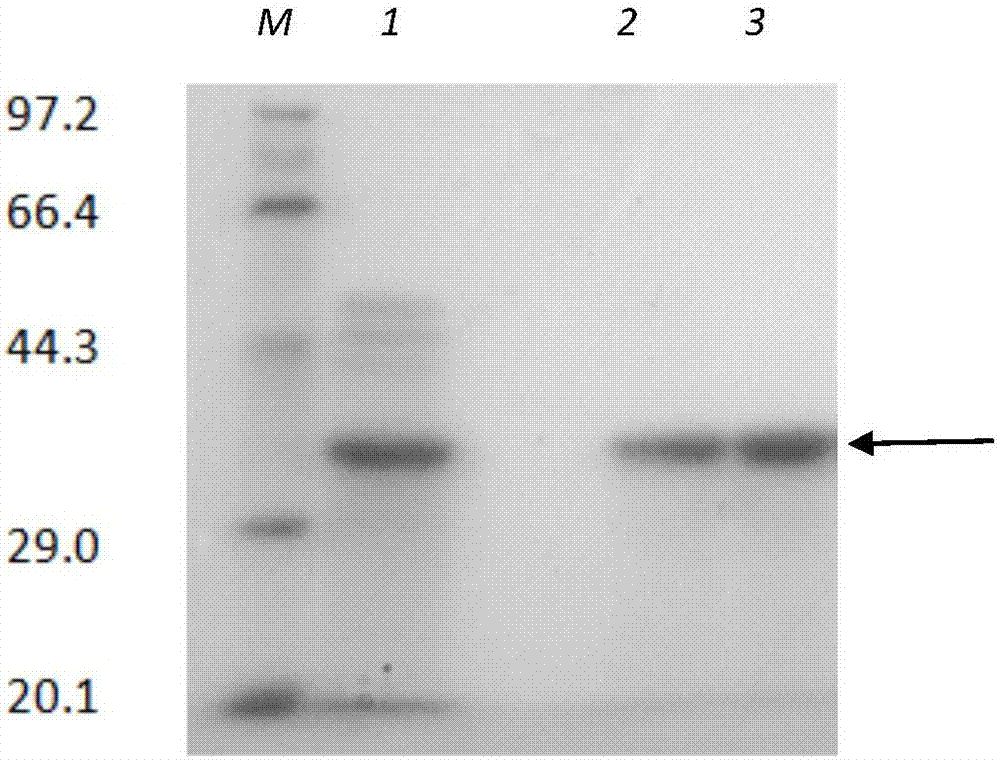
[0059] Embodiment 1: Construction and protein expression of thermophilic neutral protease engineering bacteria, comprising the following steps:
[0060] (1) Cultivation of thermophilic bacteria Thermus thermophilus HB8 and extraction of genomic DNA
[0061] The composition of 1L thermophilic bacteria culture medium is: peptone 4.0g, yeast extract 2.0g, NaCl 1.0g, Castenholz salt solution 10.0mL, distilled water 1.0L, adjust the pH value to 7.2, 1.034×10 5 Pa high pressure steam sterilization for 20min.
[0062] Castenholz salt solution: nitrilotriacetic acid 1.0g, CaSO 4 2H 2 O 0.6g, MgSO 4 ·7H 2 O 1.0g, NaCl 0.08g, KNO 3 1.03g, NaNO 3 6.89g, Na 2 HPO 4 1.11g, FeCl 3 ·6H 2 O solution (mass fraction 0.03%) 10.0mL, Nitsch's trace elements 10.0mL, distilled water 1.0L, adjust the pH to 8.2, and filter to sterilize.
[0063] Nitsch's Trace Elements: H 2 SO 4 0.5mL, MnSO 4 ·xH 2 O 2.2g, ZnSO 4 ·7H 2 O 0.5g, H 3 BO 3 0.5g, CuSO 4 ·5H 2 O 0.016g, Na 2 MoO 4...
Embodiment 2
[0092] Example 2: Properties of Recombinant Thermoneutral Protease and Mature Thermoneutral Protease
[0093] (1) Optimum reaction temperature and temperature stability
[0094] Temperature is an important factor affecting the catalytic activity of enzymes. Using casein as a substrate, the activity of the recombinant thermophilic neutral protease of the present invention is measured within the range of 50-90°C, and the relative activity of the enzyme is plotted against the temperature, such as Figure 4 It is shown that the optimum reaction temperature of the thermophilic neutral protease is 85°C, and more than 80% of the enzyme activity can be maintained in the range of 65-90°C. The activity-temperature curve of the mature thermophilic neutralase is shown in Figure 5 , its optimum reaction temperature is 75°C, and in the range of 65-85°C, the enzyme activity is maintained at a relatively high level, close to the highest activity; in the range of 50-90°C, the mature thermop...
Embodiment 3
[0104] Example 3: Analysis of Thermophilic Neutral Protease Degraded Proteins
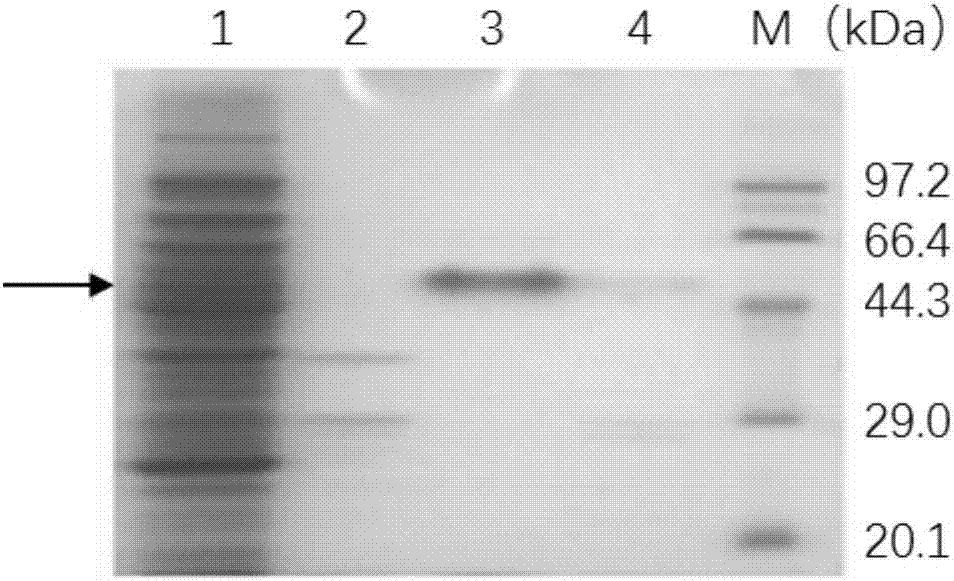
[0105] In order to detect the degradation ability of thermophilic protease on different protein substrates, 50 μL of 0.1 mg / mL thermophilic protease was mixed with 5 mg / mL casein and 2.5 mg / mL BSA respectively and incubated at 85 °C After 15min, the substrate degradation was observed by SDS-PAGE ( Figure 12 ). Electrophoresis showed that casein and BSA were all degraded within 15 minutes, indicating that the thermophilic neutral protease has strong proteolytic ability and has high application potential in the application field of protein degradation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Theoretical molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com