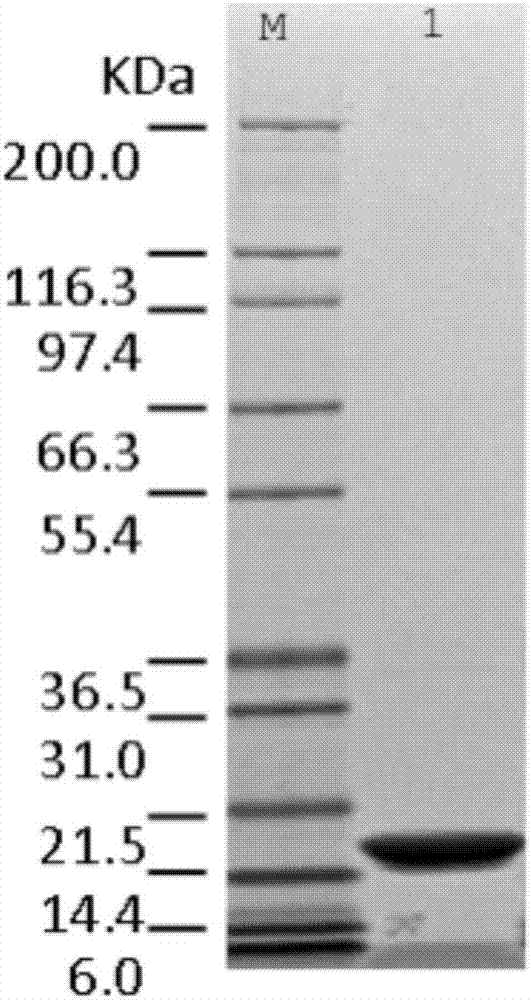
Purification method of recombinant human granulocyte colony-stimulating factor
A colony-stimulating factor and purification method technology, which is applied in the downstream protein purification field of biomedicine and bioengineering, can solve the problems of affecting the stability of protein samples and increasing the process operation time, etc., and achieves short purification cycle, shortened process time, and good feasibility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Preparation of rhG-CSF refolding solution
[0049] The bacterial strain used in the process is Escherichia coli DH5α strain transformed with pBV220 / G-CSF plasmid. After fermentation, the bacterial cells are collected, and the cells are crushed with a high-pressure homogenizer to obtain crude inclusion bodies, and then the crude inclusion bodies are washed to obtain preliminary purified inclusion bodies.
[0050] Take 30 g of initially purified inclusion bodies, and use denaturing solution (20mmol / LTris-HCl, 5mmol / L EDTA, 8mol / L Urea, 10mmol / L DTT, pH8.2 ) for denaturation and dissolution, and stirred at room temperature for 15-18 hours to obtain 600 ml of denatured protein solution. Afterwards, the protein solution is subjected to two-stage filtration and clarification at 10 μm and 0.45 μm, and then the buffer is replaced with a 5KD hollow fiber column to remove the reducing agent DTT. During the replacement process, the transmembrane pressure (TMP) is contro...
Embodiment 2
[0052] Example 2 Capto MMC purification of rhG-CSF
[0053] Adjust the pH of the refolded rhG-CSF solution to 4.0 with 0.5 mol / L hydrochloric acid, and let it stand for 10 minutes. Use a filter cartridge with a pore size of 0.22 μm for filtration.
[0054] Equilibrium: equilibrate the column with equilibrium buffer (20mmol / L citric acid / disodium hydrogen phosphate, pH4.0), flow rate 300cm / hour, until the pH is stable at 4.0;
[0055] Sample loading: load the filtered refolding liquid sample at a flow rate of 300cm / hour;
[0056] Re-equilibration: After loading the sample, equilibrate the chromatography column again with an equilibration buffer (20mmol / L citric acid / disodium hydrogen phosphate, pH4.0) at a flow rate of 300cm / hour;
[0057] Washing impurities: 10mmol / L citric acid / disodium hydrogen phosphate buffer (pH7.5) to elute impurities, flow rate 180cm / hour;
[0058] Elution: The target protein was eluted with 50 mmol / L citric acid / disodium hydrogen phosphate buffer (p...
Embodiment 3
[0059] Example 3 Screening of chromatography filler
[0060] The process of preparing rhG-CSF refolding liquid is as in Example 1. In order to obtain a chromatography medium suitable for the purification of rhG-CSF refolding solution, this study compared the ability of three fillers with weak cationic ligands to purify the refolding solution. The purification process was referred to in Example 2, and the results are shown in Table 1. HPLC purity of samples obtained with CM FF was lower than mixed mode Capto MMC and HCX, and the flow rate is slow and the yield is low. In comparison, Capto MMC and HCX can better meet the pharmacopoeia standard of rhG-CSF, and is suitable for large-scale industrial scale-up.
[0061] Table 1: Comparison of the purification effects of three fillers on rhG-CSF refolding solution
[0062]
[0063]
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com