N-diphenylphosphine oxide carbazole-9-arylfluorene sterically hindered light-emitting material and preparation method
A technology of diphenylphosphine carbazole and diphenylphosphine carbazole is applied in the field of organic light-emitting material preparation, which can solve the problems of increasing the difficulty of device fabrication, reducing device repeatability, unstable device performance and the like, and achieving a large space The effect of steric hindrance, commercialization, and simplified device structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
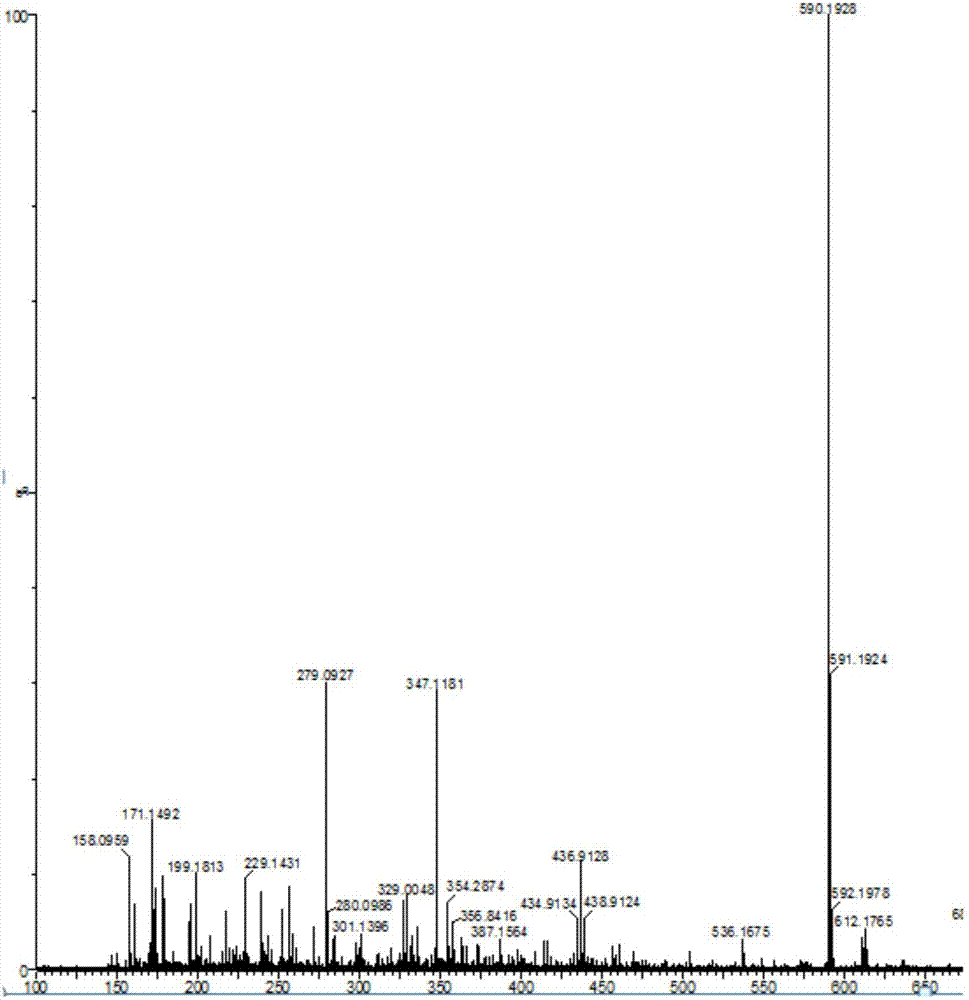
[0033] Embodiment 1: Synthesis of 3-(9-phenylfluorene)-N-diphenylphosphorcarbazole:
[0034]Take 9-hydroxy-9-phenylfluorene (0.5160g, 2mmol), N-diphenylphosphinecarbazole (0.7020g, 2mmol), dichloromethane 100mL in turn and stir to dissolve at room temperature, then add boron trifluoride diethyl ether 0.25 mL reacted for 24h. After the reaction was cooled to room temperature, about 50 mL of cold water was added to stir to quench the reaction, extracted several times with dichloromethane, and the organic phases were combined and dried over anhydrous magnesium sulfate. After drying, filter under reduced pressure, and wash the desiccant with dichloromethane, and concentrate the obtained filtrate under reduced pressure with a rotary evaporator to remove most of the solvent to obtain a concentrated crude product. Then column chromatography, with ethyl acetate and sherwood oil as detergent, after purification, white solid product 3-(9-phenylfluorene)-N-diphenylphosphocarbazole (yiel...
Embodiment 2
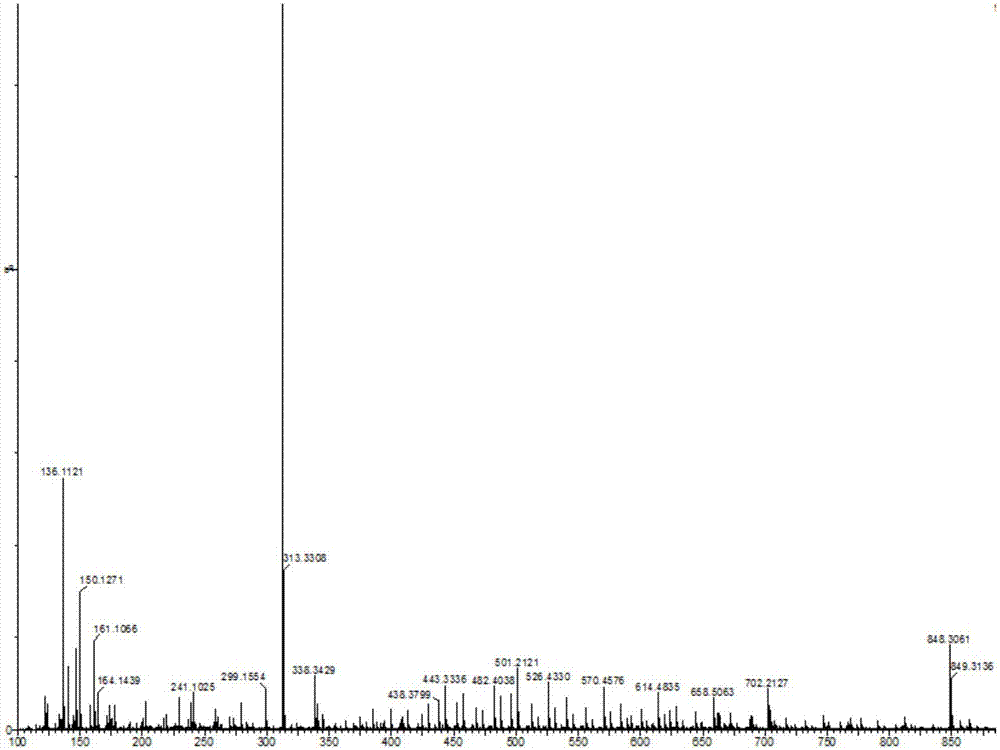
[0035] Example 2: Synthesis of 3,6-bis(9-phenylfluorene)-N-diphenylphosphorcarbazole:
[0036] Take 9-hydroxy-9-phenylfluorene (0.5160g, 2mmol), N-diphenylphosphinecarbazole (0.3510g, 1 mmol), and dichloromethane 100mL in turn, stir and dissolve at room temperature, and then add boron trifluoride diethyl ether 0.25 mL reacted for 24h. After the reaction was cooled to room temperature, about 50 mL of cold water was added to stir to quench the reaction, extracted several times with dichloromethane, and the organic phases were combined and dried over anhydrous magnesium sulfate. After drying, filter under reduced pressure, and wash the desiccant with dichloromethane, and concentrate the obtained filtrate under reduced pressure with a rotary evaporator to remove most of the solvent to obtain a concentrated crude product. Then column chromatography, with ethyl acetate and sherwood oil as detergent, after purification, white solid product 3,6-bis(9-phenylfluorene)-N-diphenylphospho...
Embodiment 3
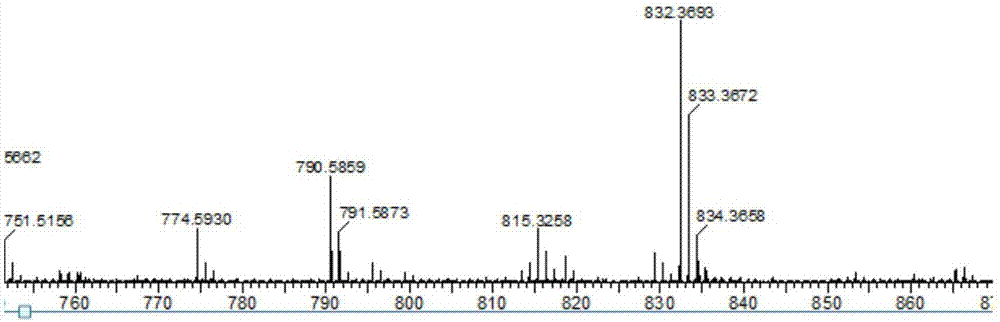
[0037] Example 3: Synthesis of 3,6-bis(9-phenylfluorene)-N-diphenylphosphoroxycarbazole:
[0038] 3,6-bis(9-phenylfluorene)-N-diphenylphosphorcarbazole (0.8320g, 1mmol) was dissolved in a solvent of ethanol and chloroform and stirred, then slowly added 1.2mL of 30% hydrogen peroxide, at room temperature Stir the reaction for 5-72 hours, extract with dichloromethane several times, combine the organic phases and dry with anhydrous magnesium sulfate; after drying, filter under reduced pressure and wash the desiccant with dichloromethane, and concentrate the obtained filtrate under reduced pressure with a rotary evaporator Most of the solvent was removed to obtain a concentrated crude product; then column chromatography, using ethyl acetate and petroleum ether as detergents, purified to obtain a white solid product 3,6-bis(9-phenylfluorene)-N-diphenyl Phosphate oxycarbazole. (Yield: 43%). LC-MS (EI) m / z 848.3061 [M + ].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com