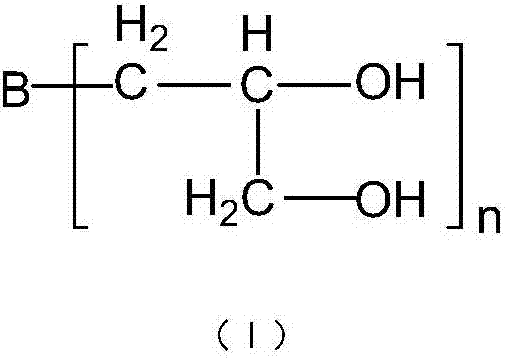
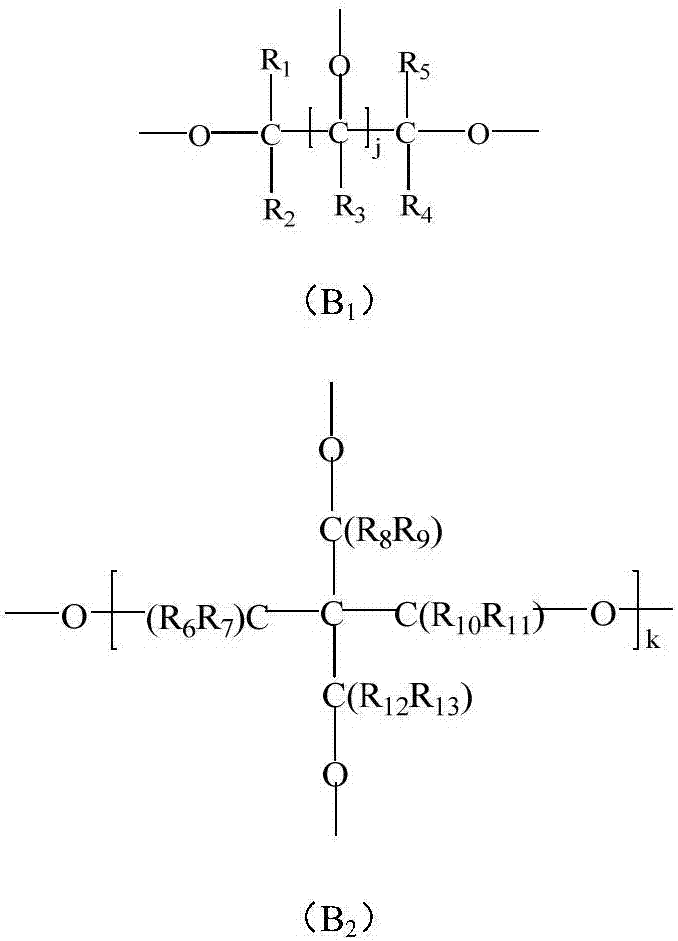
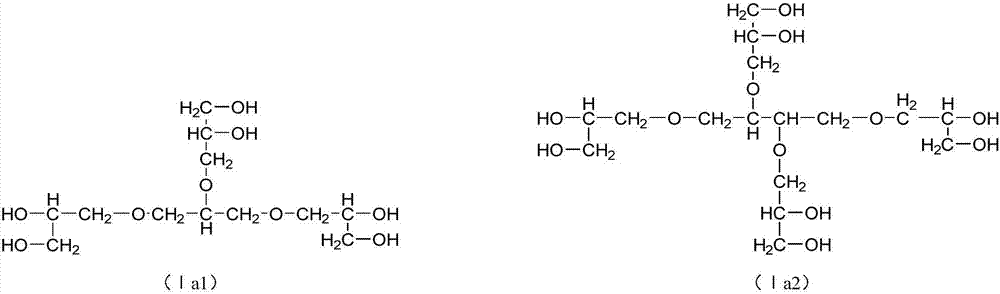
Dobby polyethylene glycol and its active derivative
A multi-arm polyethylene glycol and derivative technology, which is applied in the directions of organic active ingredients, medical preparations of non-active ingredients, organic chemistry, etc., can solve the problem of narrow molecular weight distribution, insufficient purity of multi-arm polyethylene glycol, and molecular weight distribution. leniency issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0211] Embodiment 1: synthetic glycerol triglyceride
[0212] Synthesize glycerol triglyceride ether of following structure:
[0213]
[0214] Add glycerol (0.1mol), dimethyl sulfoxide (100mL) and potassium hydroxide (0.6mol) in the there-necked flask, stir in a water bath, then drop epichlorohydrin (0.9mol) in the reaction system, control The reaction temperature did not exceed 35°C, and the reaction was carried out overnight at room temperature. After the reaction, the reaction solution was filtered, and the filter residue was washed with dichloromethane, then the filtrate was collected, and the dichloromethane was removed by rotary evaporation, and finally washed with saturated brine, extracted with ethyl acetate, and rotary evaporated to obtain a crude product. The crude product was molecularly distilled to obtain pure glycerol glycidyl ether.
[0215] The obtained glycerol glycidyl ether (1 g) was dissolved in 10 mL of purified water, and then potassium hydroxide was...
Embodiment 2
[0219] Embodiment 2: Synthesis of Tetraglycerol Tetraglycerol Ether
[0220] Synthesize tetraglycerol tetraglyceride of following structure:
[0221]
[0222] Add tetramethylene glycol (0.1mol), dimethyl sulfoxide (100mL) and potassium hydroxide (0.8mol) in the there-necked flask, stir in a water bath, then drip epichlorohydrin (1.2mol) in the reaction system, control The reaction temperature did not exceed 35°C, and the reaction was carried out overnight at room temperature. After the reaction, the reaction solution was filtered, and the filter residue was washed with dichloromethane, then the filtrate was collected, and the dichloromethane was removed by rotary evaporation, and finally washed with saturated brine, extracted with ethyl acetate, and rotary evaporated to obtain a crude product. The crude product was molecularly distilled to obtain pure butanetrol glycidyl ether.
[0223] The obtained tetramethylene glycol glycidyl ether (1 g) was dissolved in 10 mL of puri...
Embodiment 3
[0227] Embodiment 3: Synthesis of Pentapentyl Pentaglyceryl Ether
[0228] Synthesize Pentapentyl Pentaglyceryl Ether of the following structure:
[0229]
[0230] Add pentapentyl alcohol (0.1mol), dimethyl sulfoxide (100mL) and potassium hydroxide (1.0mol) in the there-necked flask, stir in a water bath, then drop epichlorohydrin (1.5mol) in the reaction system, control The reaction temperature did not exceed 35°C, and the reaction was carried out overnight at room temperature. After the reaction, the reaction solution was filtered, and the filter residue was washed with dichloromethane, then the filtrate was collected, and the dichloromethane was removed by rotary evaporation, and finally washed with saturated brine, extracted with ethyl acetate, and rotary evaporated to obtain a crude product. The crude product was molecularly distilled to obtain pure pentapentyl glycidyl ether.
[0231] The obtained pentapentyl glycidyl ether (1 g) was dissolved in 10 mL of purified w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| polydispersity index | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com