Method for preparing propylene oxide in microchannel reactor
A technology of microchannel reactor and propylene oxide, which is applied in chemical instruments and methods, chemical/physical/physical chemical reactors, organic chemistry, etc., can solve problems such as low conversion rate and selectivity, and explosion hazard, and achieve High conversion rate and selectivity, improved utilization, and short diffusion path
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
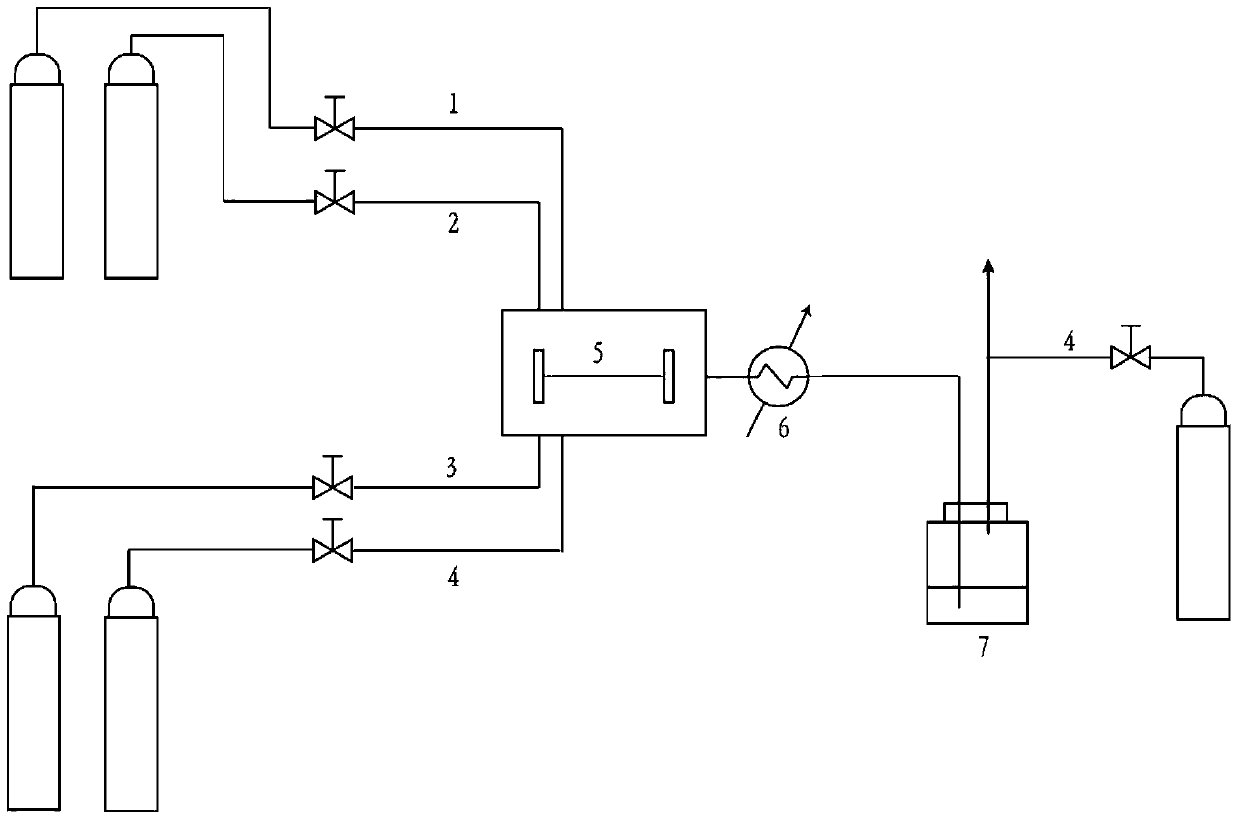
[0029] The propylene oxide synthesis method uses hydrogen, oxygen, and propylene as gas phase raw materials, nitrogen as a diluent gas, and does not use a liquid phase carrier. The microchannel reactor is made of polymethyl methacrylate. The cross section of the microchannel is square, with a side length of 200 microns and a length of 1 cm. The amount is 0.05% (w / w).
[0030] The whole set of equipment is at room temperature (about 20°C). The gas source in the laboratory test is a gas cylinder. The gas is decompressed to 0.1MPa through a pressure reducing valve, and the flow is controlled by a mass flow meter. Then enter the microchannel chip reaction unit. The product flowing out from the chip is separated from gas and liquid after cooling. The flow rates of the four gases in the gas phase entering the reaction zone are H 2 :O 2 :C 3 h 6 :N 2 =5sccm:5sccm:5sccm:10sccm, the corresponding volume ratio is 20:20:20:40. Where sccm is milliliters per minute under standard c...
Embodiment 2
[0033] According to the conditions and steps described in Example 1, the microchannel reactor is made of polymethyl methacrylate, the microchannel section is rectangular, 500 microns long, 200 microns wide, and 1 cm in length, and the inside is completely filled with The nano-Au catalyst loaded on the TS-1 carrier, the loading amount is 1% (w / w).
[0034] The whole set of equipment is at 100°C. The gas source in the laboratory test is a gas cylinder. The gas is decompressed to 0.5MPa through a pressure reducing valve, and the flow is controlled by a mass flow meter. Channel Chip Reaction Unit. The product flowing out from the chip is separated from gas and liquid after cooling. The flow rates of the four gases in the gas phase entering the reaction zone are H 2 :O 2 :C 3 h 6 :N 2 =5sccm:5sccm:5sccm:10sccm, the corresponding volume ratio is 20:20:20:40.
[0035] The conversion rate of propylene was 8.2% and the selectivity of propylene oxide was 91.5% as measured by an o...
Embodiment 3
[0037] According to the conditions and steps described in Example 1, the microchannel reactor is made of stainless steel grade SS316L. The cross section of the microchannel is an equilateral triangle with a side length of 200 microns and a length of 1 cm. The interior is completely filled with TS- 1 Nano-Au catalyst on a carrier with a loading of 5% (w / w).
[0038] The whole set of equipment is at about 300°C. The gas source in the laboratory test is a gas cylinder. The gas is decompressed to 1MPa through a pressure reducing valve, and the flow is controlled by a mass flow meter. Channel Chip Reaction Unit. The product flowing out from the chip is separated from gas and liquid after cooling. The flow rates of the four gases in the gas phase entering the reaction zone are H 2 :O 2 :C 3 h 6 :N 2 =5sccm 5sccm:5sccm:10sccm, the corresponding volume ratio is 20:20:20:40.
[0039] The conversion rate of propylene was 5.6% and the selectivity of propylene oxide was 84.3% as me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com