Phospholipid-glycosaminoglycan biomimetic extracellular matrix nanomembrane and its preparation method and application
A glycosaminoglycan and extracellular matrix technology, which is applied in the field of phospholipid-glycosaminoglycan biomimetic extracellular matrix nanomembrane and its preparation, can solve problems such as unfavorable capillary formation, and achieves the promotion of wound healing and high anticoagulant activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
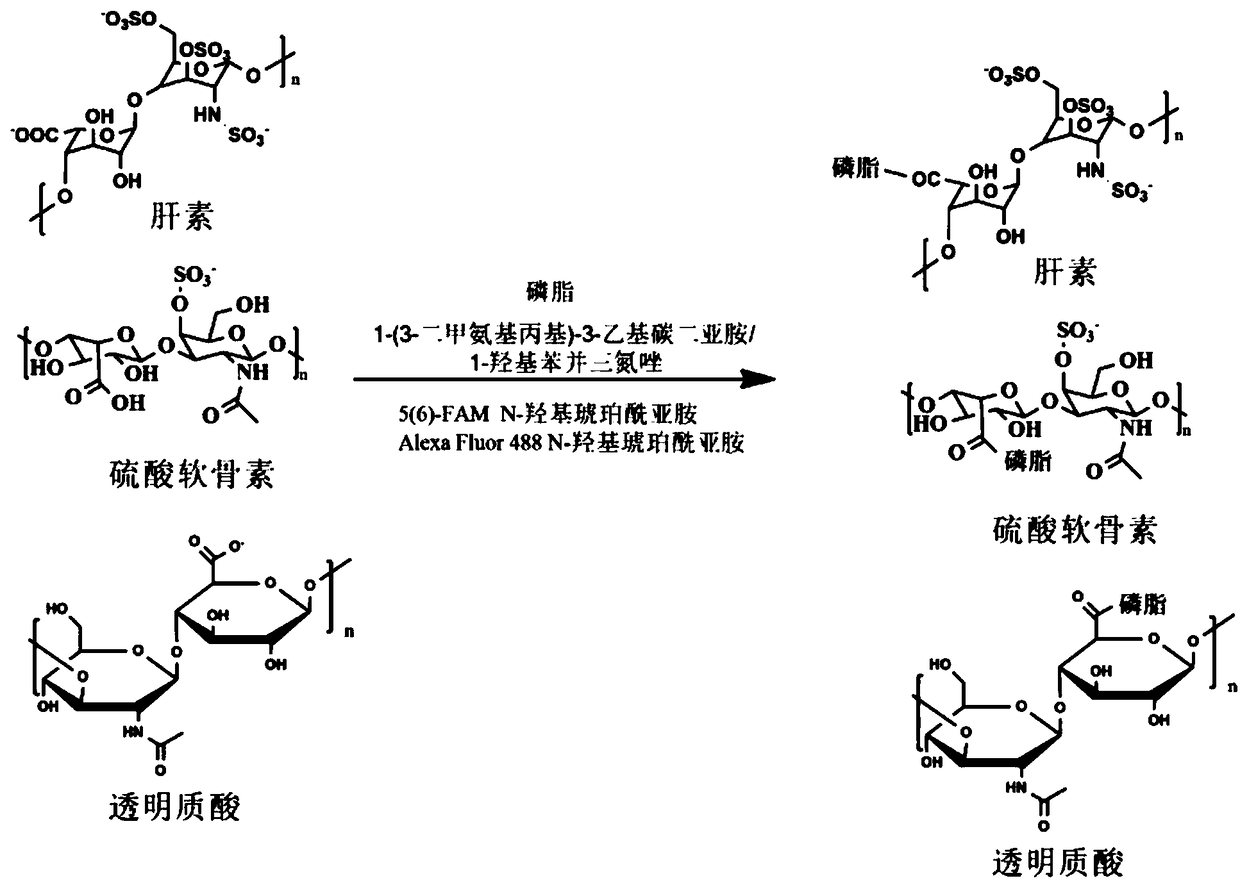
[0030] The invention provides a method for preparing a biomimetic extracellular matrix nano-membrane, comprising the following steps:
[0031] S1: Dissolve glycosaminoglycan in phosphate buffer solution, then add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide and additives to obtain a mixed solution, and then remove oxygen from the mixed solution Post-activation; wherein, the additive is 1-hydroxybenzotriazole or N-hydroxysuccinimide;
[0032] Preferably, the molar ratio of glycosaminoglycan, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide and additive is 1:1:(1~10); Including one or more of heparin, hyaluronic acid and chondroitin sulfate, the relative molecular mass of glycosaminoglycan is 8000-14000; dissolving glycosaminoglycan in phosphate buffer solution specifically includes: dissolving glycosaminoglycan Sugar is added to the phosphate buffer solution to obtain a mixture of glycosaminoglycans and phosphate buffer solution, and then ultrasonicated at 0-37°C for 10-30 minut...
Embodiment 1
[0047] This embodiment provides a biomimetic extracellular matrix nano-membrane and a preparation method thereof, the preparation process comprising:
[0048] S1: Dissolve heparin with a relative molecular mass of 12,000 in a phosphate buffer solution with a pH value of 6.4 to obtain a mixture of heparin and phosphate buffer solution, wherein, in the mixture of heparin and phosphate buffer solution, the mass fraction of heparin 30%. Sonicate the mixture of heparin and phosphate buffer solution at 25°C for 20min with a power of 200W to remove the oxygen in the reaction vessel, then add 1-(3-dimethylaminopropyl)-3-ethyl Carbodiimide and 1-hydroxybenzotriazole to obtain a mixed solution, remove oxygen from the mixed solution, and activate it at 25°C for 30 minutes, wherein heparin, 1-(3-dimethylaminopropyl)-3- The molar ratio of ethylcarbodiimide and 1-hydroxybenzotriazole was 1:1:5.
[0049] S2: Double-distilled water and organic solvents are prepared into a compound emulsion,...
Embodiment 2
[0054] This embodiment provides a biomimetic extracellular matrix nano-membrane and a preparation method thereof, the preparation process comprising:
[0055]S1: Dissolve hyaluronic acid with a relative molecular mass of 8000 in a phosphate buffer solution with a pH value of 6.0 to obtain a mixture of hyaluronic acid and phosphate buffer solution, wherein the mixture of hyaluronic acid and phosphate buffer solution In, the mass fraction of hyaluronic acid is 10%. Sonicate the mixture of hyaluronic acid and phosphate buffer solution at 5°C for 10 minutes, the ultrasonic power is 400W, and remove the oxygen in the reaction vessel, and then add 1-(3-dimethylaminopropyl)-3- Ethylcarbodiimide and 1-hydroxybenzotriazole to obtain a mixed solution, remove oxygen from the mixed solution, and activate it at 5°C for 15 minutes, wherein hyaluronic acid, 1-(3-dimethylaminopropyl )-3-ethylcarbodiimide and 1-hydroxybenzotriazole in a ratio of 1:1:1.
[0056] S2: Double-distilled water and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com