Tumor-targeted double-drug-delivery nano carrier and preparation method thereof
A nanocarrier, tumor targeting technology, applied in the field of biomedical engineering materials, can solve the problems of uncontrollable drug release, low tumor specificity, high cytotoxicity, etc., to improve drug effect, low cytotoxicity, tumor targeting strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Preparation of dihydroxy polycaprolactone (PCL) with disulfide bonds
[0040] Dissolve cystamine and ε-caprolactone with disulfide bonds in dry toluene, slowly add the catalyst stannous isooctanoate dropwise for 20 minutes under anhydrous and oxygen-free conditions, and condense at 110°C in a nitrogen environment after the dropwise addition Reflux reaction for 18 hours, magnetic stirring during the reaction, and the rotation speed was 300rpm; after the reaction was completed, the toluene solvent was removed by rotary evaporation at 40°C, and then precipitated with cold ether, and then vacuum-dried at 40°C for 12h to obtain dihydroxypolycaprolactone with a disulfide bond (PCL).
[0041] Wherein, the quality of cystamine is 200mg; The total volume of toluene is 10mL; The mol ratio of cystamine, ε-caprolactone, stannous isooctanoate is 1:132:0.05; The volume ratio of cold ether and reaction system solution is 10 :1.
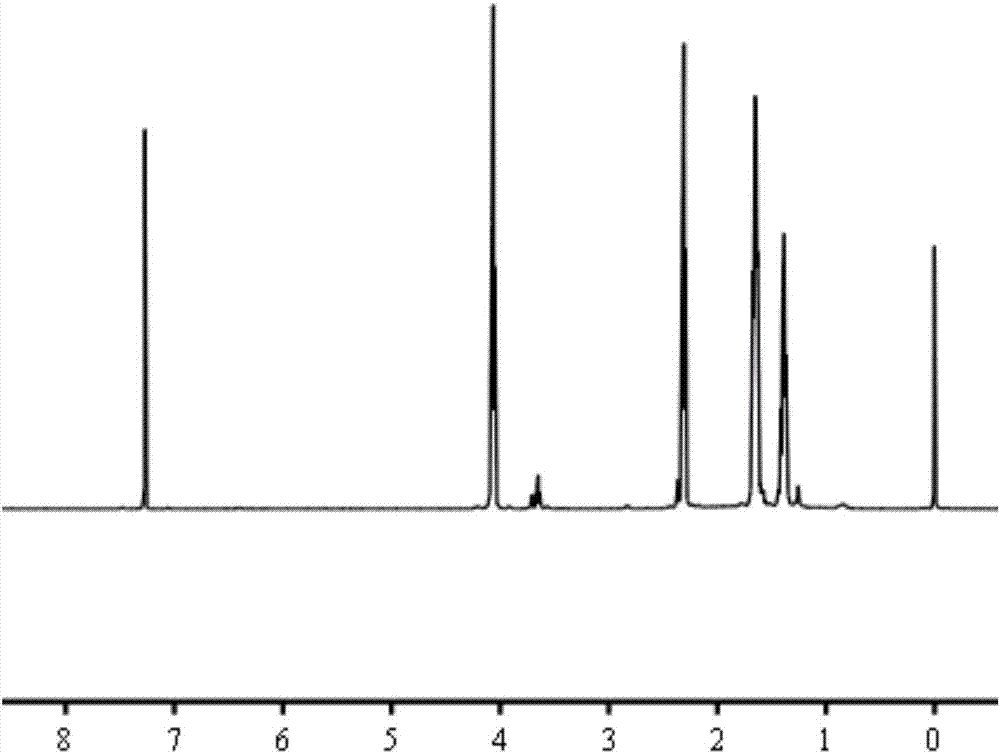
[0042] The prepared dihydroxy polycaprolac...
Embodiment 2
[0043] Example 2: Preparation of acrylate-terminated dihydroxypolycaprolactone (PCL-Acrylate):
[0044] Dissolve triethylamine and acryloyl chloride in dry toluene, respectively, and first vacuum and blow nitrogen into the dihydroxy polycaprolactone (PCL) having a disulfide bond prepared in Example 1, and then successively add triethylamine and The toluene solution of acryloyl chloride was slowly added dropwise for 30 minutes in an ice bath and protected from light; condensed and refluxed at 80°C for 8 hours, magnetically stirred at a speed of 300rpm during the reaction, after the reaction, the reaction solution was filtered to remove triethylamine hydrochloride After crystallization, the filtrate was precipitated in excess cold n-hexane, filtered by suction, and the white powder product was collected, and finally vacuum-dried at 40°C for 24 hours to obtain acrylate-terminated dihydroxy polycaprolactone (PCL-Acrylate).
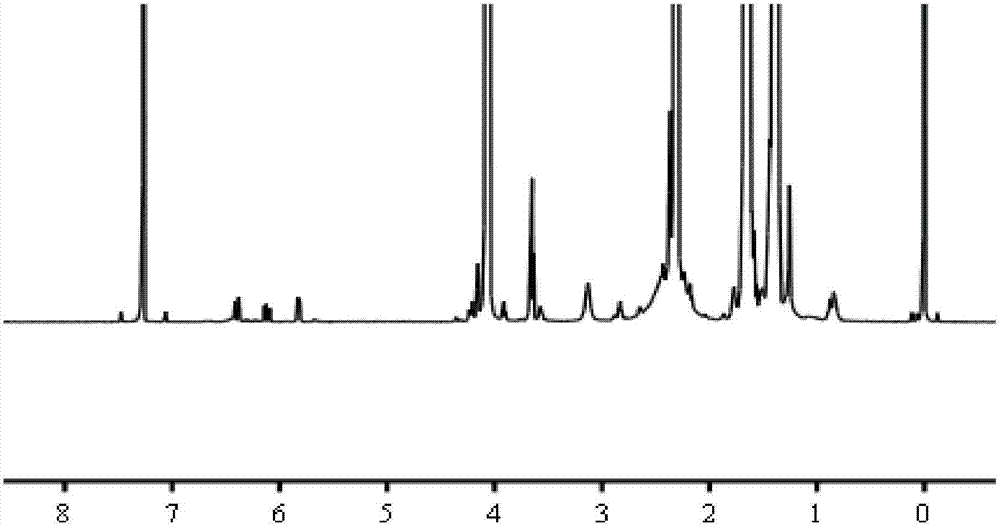
[0045] Wherein, the quality of the dihydroxy polycaprola...
Embodiment 3
[0047] Example 3: Preparation of polycaprolactone-poly(amide-amine) polymer (PCL-PAMAM)
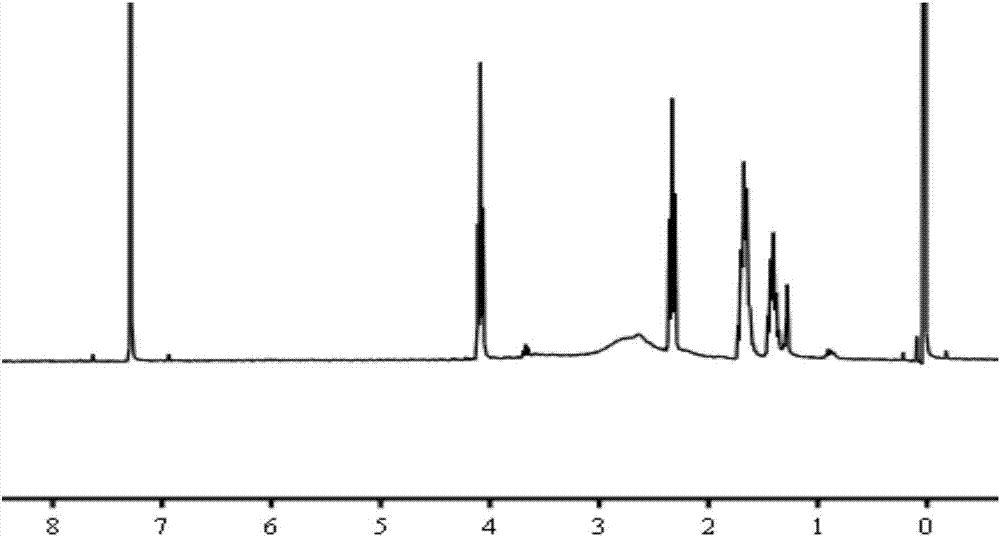
[0048] The polyamide-amine dendrimer (PAMAM) and the acrylate-terminated bishydroxypolycaprolactone (PCL-Acrylate) prepared in Example 2 were dissolved in chloroform respectively, and the polyamide-amine dendrimer (PAMAM) was completely dissolved at 55°C under a nitrogen atmosphere, and then the chloroform solution of acrylate-terminated bishydroxypolycaprolactone (PCL-Acrylate) was slowly added dropwise to the polyamide-amine dendrimer ( In the chloroform solution of PAMAM), vacuumize, nitrogen flow, reflux reaction at 55 ℃ for 24h, magnetic stirring during the reaction, the rotating speed is 300rpm; Days, dialyze unreacted monomers, and precipitate with excess cold petroleum ether to obtain a white precipitate, dissolve it with a small amount of dichloromethane, slowly add it dropwise to deionized water, stir overnight, evaporate the organic solvent, and freeze-dry After 48 hours, the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com