Amylosucrase and method for using same to produce Alpha-arbutin by transformation
A technology of amylosucrase and arbutin, which is applied in the field of food biology, can solve the problems of complicated purification, many steps, expensive conversion rate of substrates, etc., and achieves the effects of low demand, short conversion time and good industrial application potential.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Construction of recombinant expression vector pET-CC-AS and acquisition of genetically engineered bacteria BL21(DE3) / pET-CC-AS:
[0029] Synthesized with Nde I / Xho I restriction sites Cellulomonas carboniz The 1935 bp of the amylosucrase gene of T26 was ligated into pET-22b(+) to obtain the pET22-CC-AS plasmid.
[0030] Add 5 μL pET22-CC-AS plasmid (40ng / μL) to 100 μL BL21 competent cell suspension, and mix gently.
[0031] Let it stand in an ice bath for 30 minutes. After the plasmid is transformed, evenly spread it on an LB plate containing 100 μg / mL ampicillin, and culture it upside down at 37°C for 12 hours to obtain a positive clone, which is named recombinant Escherichia coli BL21(DE3) / pET22-CC-AS .
Embodiment 2
[0032] Example 2 Recombination Cellulomonas carboniz Acquisition of T26 enzyme
[0033] Single clones of the genetically engineered strain BL21(DE3) / pET-CC-AS were picked to LB seed medium containing 50 μg / mL ampicillin, and cultured at 37°C with shaking at 200 rpm for 12 hours.
[0034] The seed culture solution was added to the fermentation medium at a ratio of 1:50 and cultivated to OD at 37°C 600 0.6-0.8, add IPTG to a final concentration of 1mM;
[0035] Induce the expression at 28°C and 200rpm for 6h, collect the bacterial liquid; centrifuge at 4°C and 10000rpm for 10min to collect the bacterial cells, discard the supernatant, and wash with normal saline for 3 times;
[0036] After centrifuging to obtain the cells, ultrasonically disrupt them, add the equilibrium buffer at a ratio of 1 g of cells: 20 mL of equilibrium buffer, ultrasonically disrupt in an ice bath, and centrifuge the supernatant to obtain purified amylosucrase by metal affinity chromatography. The low...
Embodiment 3
[0040] Example 3 Efficient preparation of α-arbutin
[0041] The molar concentration ratio of substrate sucrose:hydroquinone is 1:1 to configure the reaction solution, and amylosucrase is added to it for catalytic conversion. The conversion reaction conditions are: the concentration range of the substrate (sucrose, hydroquinone) is 1 %~30%, the dosage of enzyme is 1~3U / mg hydroquinone, pH 5.5~7.0, the conversion reaction temperature is 35~45℃, the conversion reaction time is 3~4h, and the α- The reaction liquid of arbutin.
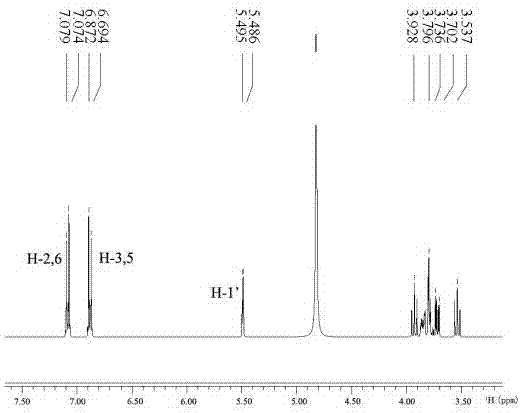
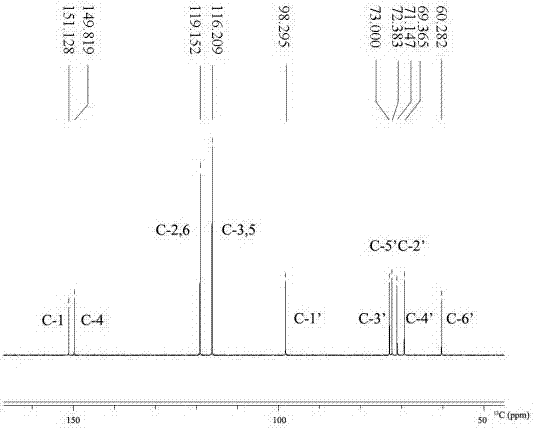
[0042]After separating and purifying the product in the reaction solution by HPLC, use AVANCE III 400MHz digital
[0043] NMR spectrometer (Broker Biospin International AG) nuclear magnetic instrument, two pictures were obtained by analysis, figure 1 is the hydrogen spectrum, figure 2 It is a carbon spectrogram, the peak position marked in the figure is exactly the same as that of the α-arbutin standard product, and the reaction product is determined ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com