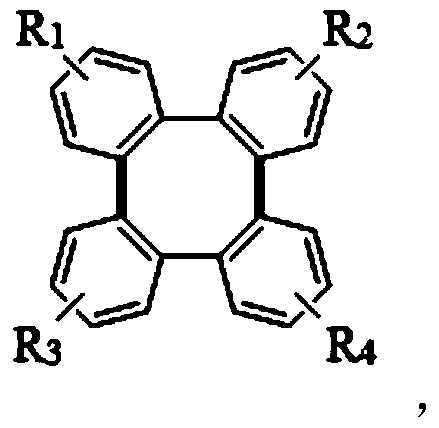
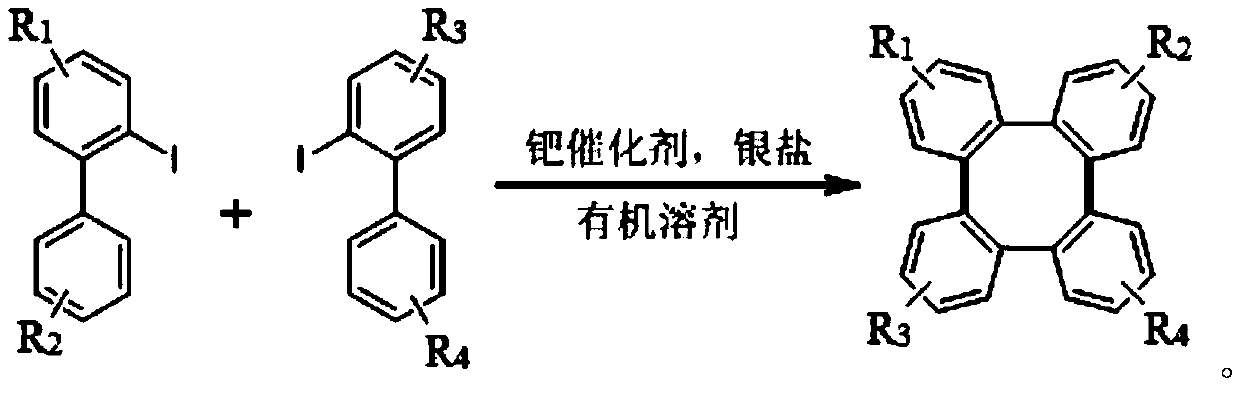
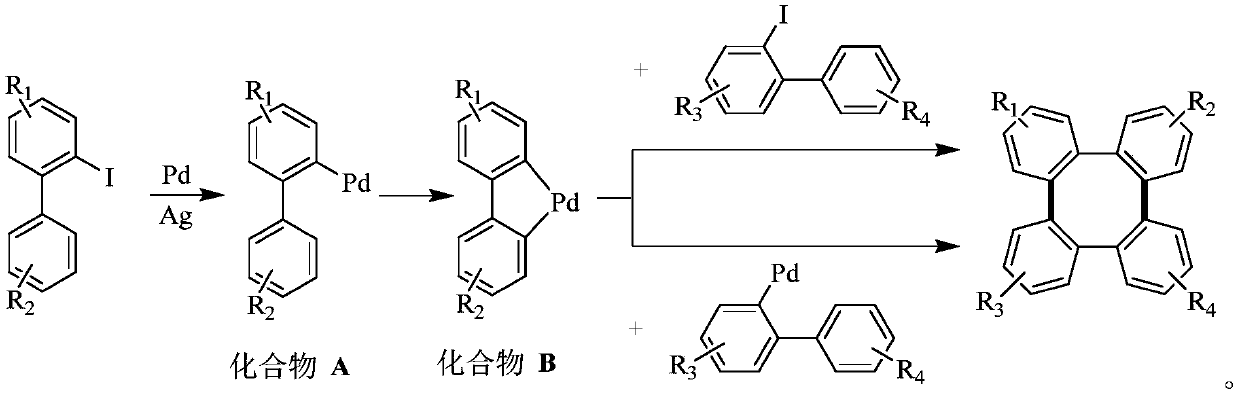
A kind of synthetic method of tetraphenylene compound
A synthesis method and compound technology are applied in the synthesis field of tetraphenylene compounds, which can solve the problems of many reaction steps and low production efficiency, and achieve the effects of short reaction steps, simple operation and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~16
[0033] Add 2-iodobiphenyl, palladium catalyst palladium acetate, silver salt, and trifluoroacetic acid into the reaction tube, and stir at 100°C; after a period of reaction, cool the reactant to room temperature, add ethyl acetate to it for extraction, and extract The organics gathered together were filtered with diatomaceous earth; saturated sodium bicarbonate solution was added to the filtrate to wash until the pH of the aqueous phase was neutral; the collected organic phase was dried with anhydrous sodium sulfate and concentrated under reduced pressure at 40°C. After separation by column chromatography (petroleum ether, elution), the obtained white solid is tetraphenylene (1a):
[0034]
[0035] 1 H NMR (400MHz, CDCl 3 ):δ7.29(m,8H),7.18(m,8H);
[0036] 13 C NMR (100MHz, CDCl 3 ): δ141.51, 129.00, 127.21;
[0037] HRMS(ESI-TOF)m / z:calcd for C 24 h 16 Na + :327.1144(M+Na) + ,found: 327.1142..
[0038] Note: the palladium acetate used in the above examples was pu...
Embodiment 7
[0043] For Example 6 and Example 7, Ag 2 CO 3 and Ag 2 The amount of O is 0.3mmol, which is equivalent to 0.6mmol of Ag, which shows that the addition of too much Ag will inhibit the oxidative addition of palladium to the carbon-iodine bond of the substrate, resulting in the inability of the reaction to proceed downward. For Example 14, the reaction cannot proceed without adding catalyst palladium, indicating that palladium is necessary.
Embodiment 17~21
[0045] Replace the reaction substrate with 2-iodobiphenyl compounds with substituents, palladium acetate, silver carbonate, trifluoroacetic acid, and stir at 100°C; after a period of reaction, cool the reactant to room temperature, and add acetic acid to it Ethyl extraction, the organic matter gathered together after extraction and filtered with diatomaceous earth; Add saturated sodium bicarbonate solution to the filtrate and wash until the pH of the aqueous phase is neutral; After the collected organic phase is dried with anhydrous sodium sulfate, the Concentrate under reduced pressure at 40°C, and separate by column chromatography (petroleum ether, elution), and the obtained white solid is tetraphenylene compound (2a, 4a, 5a, 6a). Investigate the impact of different 2-iodobiphenyl compounds on the synthetic method of the present invention, its concrete reaction formula can be expressed as:
[0046]
[0047] Wherein, R is fluorine, chlorine, methyl or phenyl.
[0048] The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com