Preparation method of herbicide triclopyr butoxyethyl ester
A technology of clopyroxyethyl butoxyethyl ester and clopyroxyacetic acid, which is applied in the field of agricultural chemicals, can solve the problems of non-compliance with green pesticide process requirements, non-compliance with green preparation requirements, cumbersome operations, etc., and achieve cost Low, little pollution, and the effect of improving the reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
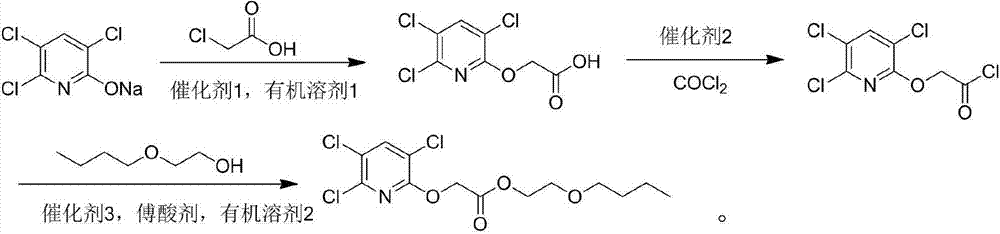
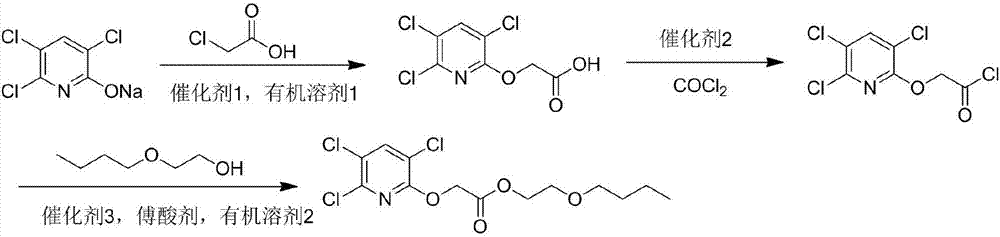
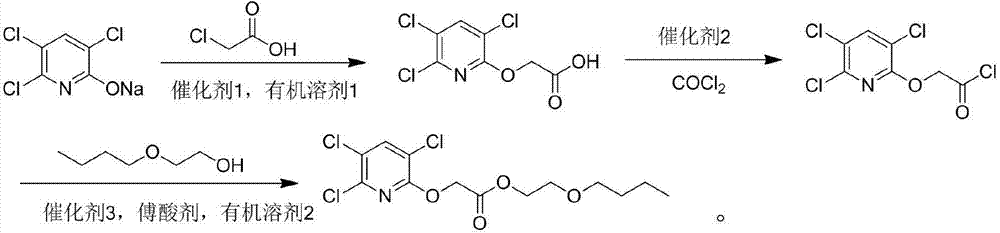
[0029] Add 22g (0.1mol) sodium triclopyridinate and 100mL of N,N-dimethylformamide into a three-necked reaction flask equipped with a motor, condenser and dropping funnel, and add 0.2g of phase transfer catalyst PEG-400 under stirring , then dropwise add 20mL N,N-dimethylformamide liquid containing 9.4g (0.1mol) chloroacetic acid, after the addition, heat up to 140°C, react for 4.0h, cool, add 400mL ice water, acidify with dilute hydrochloric acid to pH=2 -3, extracted 4 times with 100mL ethyl acetate, combined the organic layers, washed twice with 100mL saturated brine, dried the organic layer, and desolventized to obtain the product. After recrystallization from ethanol, triclopyr was a white powder with a content of 98.2%. The rate is 95.3%.
[0030] Add 25.4g (0.1mol) of clopyroxyacetic acid, 80mL of dichloromethane, 0.3mol of oxalyl chloride into a dry one-necked bottle, and add a catalytic amount of N,N-dimethylformamide dropwise. React overnight at room temperature, re...
Embodiment 2
[0033] Add 22g (0.1mol) sodium triclopyridinate and 100mL of N,N-dimethylacetamide into a three-necked reaction flask equipped with a motor, condenser and dropping funnel, and add 0.2g of phase transfer catalyst PEG-400 under stirring , then add dropwise 20mL of N,N-dimethylacetamide liquid containing 9.4g (0.1mol) of chloroacetic acid, heat up to 140°C after addition, react for 5.0h, cool down, add 400mL of ice water, acidify with dilute hydrochloric acid to pH=2 -3, extracted 4 times with 110mL ethyl acetate, combined the organic layers, washed twice with 100mL saturated brine, dried the organic layer, and desolventized to obtain the product, after ethanol recrystallization, white powder triclopyr, with a content of 96.3%, was obtained. The rate is 92.3%.
[0034] Add 25.4g (0.1mol) of clopyroxyacetic acid, 80mL of dichloromethane, 0.3mol of oxalyl chloride into a dry one-necked bottle, and add a catalytic amount of N,N-dimethylformamide dropwise. React overnight at room te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com