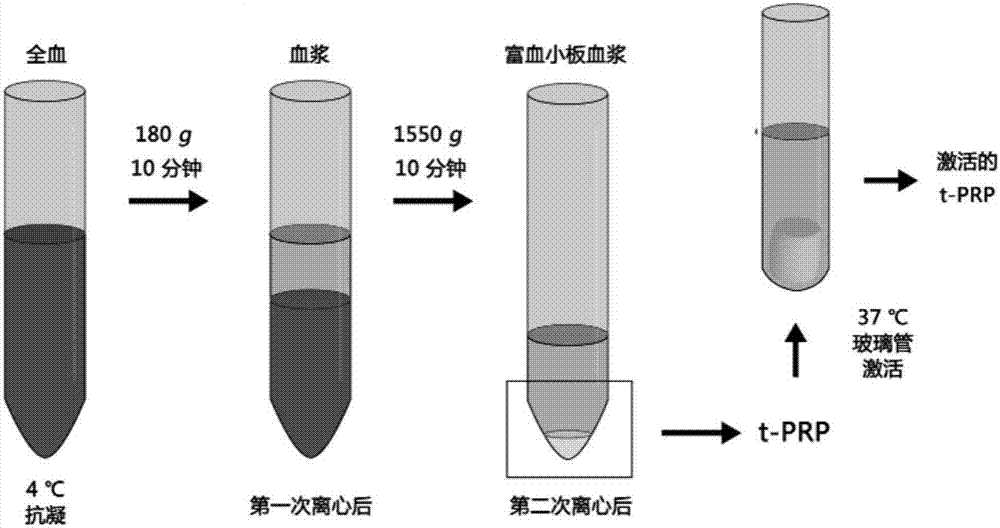
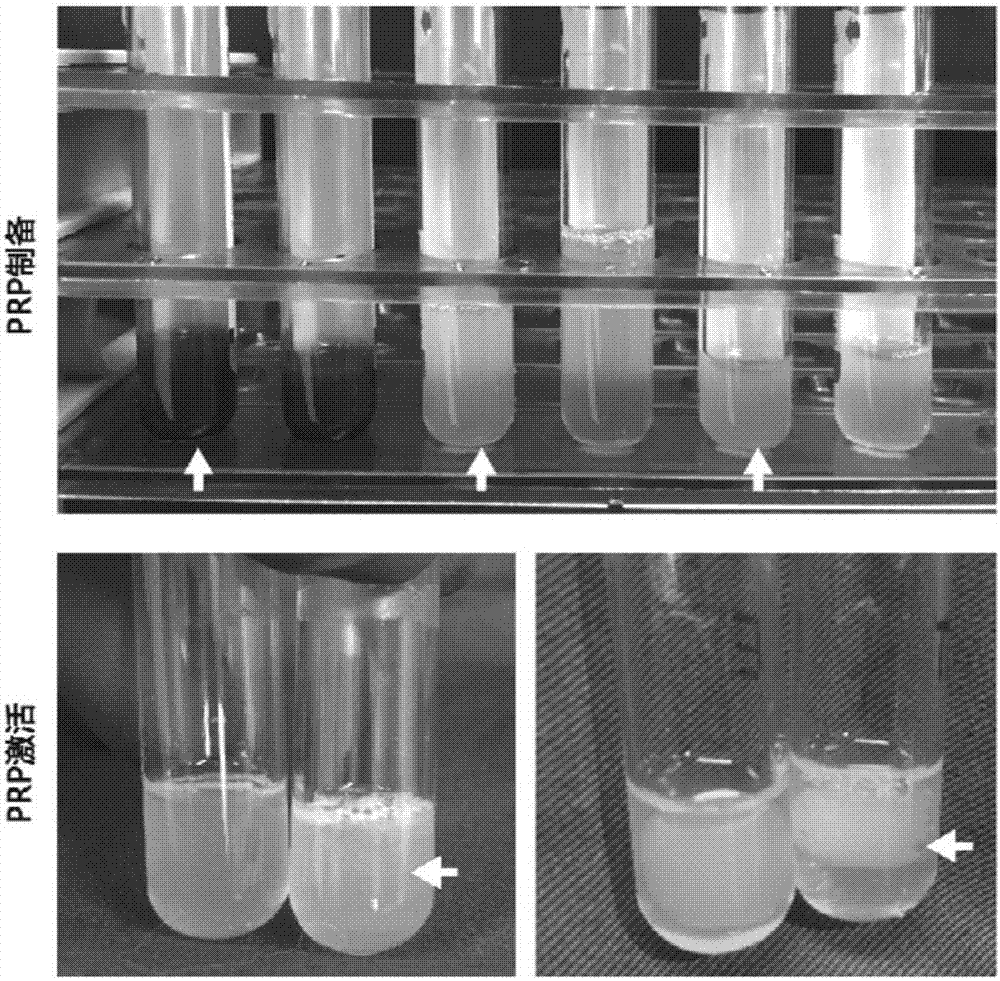
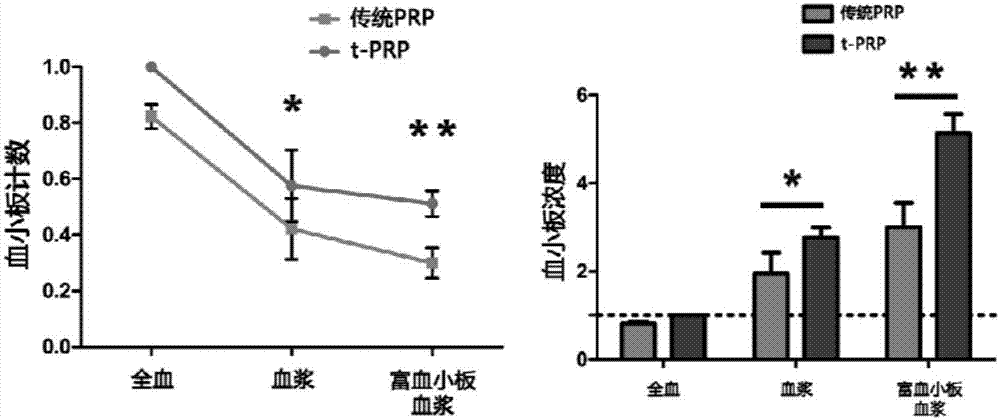
Rich-blood-platelet plasma preparation method without exogenous additives
A technology of platelet-rich plasma and additives, which is applied in the field of platelet-rich plasma preparation without exogenous additives, can solve problems such as inability to obtain PRP accurately, destroy platelets, and drop blood pressure, so as to avoid clinical adverse reactions, ensure accuracy, Guaranteed Effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0031] The method will be further described below in conjunction with the accompanying drawings and specific embodiments. Consumables, equipment, etc. used in the implementation, unless otherwise specified, are materials that can be obtained from commercial sources.
[0032] The prepared PRP is finally required to be of clinical grade and can be used by humans. The following whole process is strictly required to be aseptic, and all the instruments used need to be sterilized by high temperature, high pressure or gas.
[0033] PRP preparation experiment control example (conventional secondary centrifugation method)
[0034] Main equipment and consumables: Eppendorf Centrifuge 5810R centrifuge, Sysmex Xe-2100hematology analyzer automatic blood cell analyzer, Corning 15ml plastic centrifuge tube, BD Vacutainer R K3EDTA anticoagulant tube, No. 7 blood collection needle, 60ml syringe, ACD-A anticoagulant, thrombin.
[0035] Blood inclusion criteria: 1) Venous blood blood cell anal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com