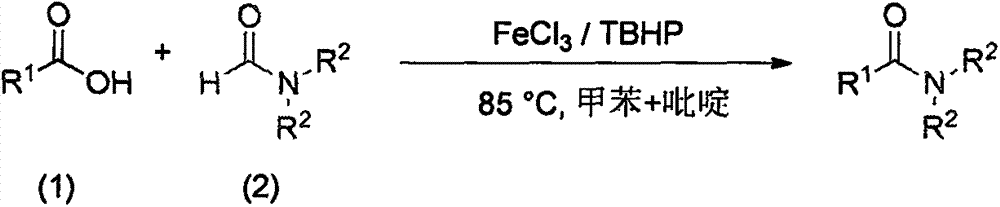
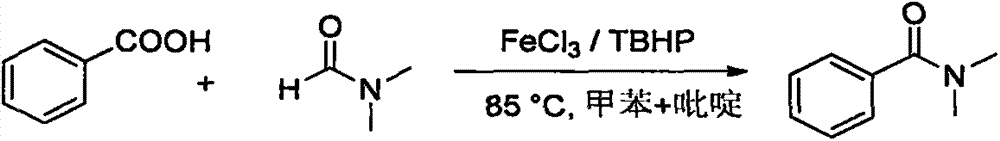
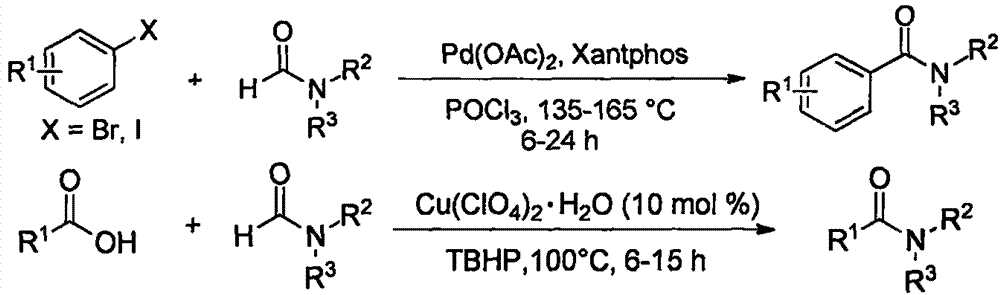FeCl3 catalyzed amide compound synthesis method
A technology for amide compounds and synthesis methods, applied in chemical instruments and methods, preparation of organic compounds, formation/introduction of amide groups, etc., can solve the problems of lack of environmental protection and economy, high toxicity, high odor, etc. Atomic economy, low toxicity, stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Synthesis of N,N-dimethylbenzamide from benzoic acid and DMF:
[0022]
[0023] Add benzoic acid (0.036g, 0.3mmol), FeCl 3 (0.0049g, 0.03mmol), TBHP (0.116g, 0.9mmol, 70% aqueous solution), DMF (1mL), toluene (1mL), pyridine (0.118g, 1.5mmol), fill it with argon, tighten the bottle cap , external temperature 85 ℃ airtight reaction 8h; gas chromatography monitoring; after the reaction was completed, the solvent was removed by rotary evaporation, and a colorless transparent liquid was obtained after separation by column chromatography (ethyl acetate:petroleum ether=4:1), with a yield of 89% .
[0024] 1 H NMR (300MHz, CDCl 3 )δ7.39(s, 5H), 3.10(s, 3H), 2.96(s, 3H); 13 C NMR (75MHz, CDCl 3 )δ171.76(s), 136.34(s), 129.61(s), 128.43(s), 127.11(s), 39.69(s), 35.43(s); MS(70eV, EI) m / z(EI) C 9 h 11 NO[M]: 149.19, 51(36), 77(100), 105(29), 148(56), 149(5).
Embodiment 2
[0026] Synthesis of N,N-dimethyl-2-chlorobenzamide from 2-chlorobenzoic acid and DMF:
[0027]
[0028] Add 2-chlorobenzoic acid (0.047g, 0.3mmol), FeCl 3 (0.0049g, 0.03mmol), TBHP (0.116g, 0.9mmol, 70% aqueous solution), DMF (1mL), toluene (1mL), pyridine (0.118g, 1.5mmol), fill it with argon, tighten the bottle cap , closed reaction at an external temperature of 85° C. for 8 h; gas chromatography monitoring; after the reaction was completed, the solvent was removed by rotary evaporation, and a white solid was obtained after separation by column chromatography (ethyl acetate:petroleum ether=4:1), with a yield of 92%.
[0029] 1 H NMR (300MHz, CDCl 3 )δ7.35-7.19 (m, 4H), 3.07 (s, 3H), 2.80 (s, 3H); 13 C NMR (75MHz, CDCl 3 )δ168.57(s), 136.43(s), 130.28(d, J=14.4Hz), 129.67(s), 127.85(s), 127.29(s), 38.18(s), 34.76(s); MS( 70eV, EI)m / z(EI)C 9 h 10 ClNO [M]: 183.63, 75(100), 111(84), 139(59), 182(16), 184(6).
Embodiment 3
[0031] Synthesis of N,N-dimethyl-2-iodobenzamide from 2-iodobenzoic acid and DMF:
[0032]
[0033] Add 2-iodobenzoic acid (0.074g, 0.3mmol), FeCl 3 (0.0049g, 0.03mmol), TBHP (0.116g, 0.9mmol, 70% aqueous solution), DMF (1mL), toluene (1mL), pyridine (0.118g, 1.5mmol), fill it with argon, tighten the bottle cap , closed reaction at an external temperature of 85° C. for 8 h; gas chromatography monitoring; after the reaction was completed, the solvent was removed by rotary evaporation, and a light yellow solid was obtained after separation by column chromatography (ethyl acetate:petroleum ether=4:1), with a yield of 95%.
[0034] 1 H NMR (300MHz, CDCl 3 )δ7.81(dd, J=8.0, 0.8Hz, 1H), 7.38(td, J=7.5, 1.1Hz, 1H), 7.21(dd, J=7.6, 1.6Hz, 1H), 7.09-7.03(m , 1H), 3.13(s, 3H), 2.84(s, 3H); 13 C NMR (75MHz, CDCl 3 )δ170.8, 142.9, 139.1, 130.2, 128.5, 127.1, 92.5, 38.5, 34.8; MS (70eV, EI) m / z (EI) C 9 h 10 INO, [M]: 275.09, 50(100), 76(91), 91(14), 148(11), 231(5), 274(20), 275...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com