A kind of purification method of lentivirus
A purification method, lentivirus technology, applied in the direction of viruses, biochemical equipment and methods, recovery/purification, etc., to achieve the effects of easy amplification, good repeatability and stability, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Optimization of virus loading by membrane chromatography purification method.
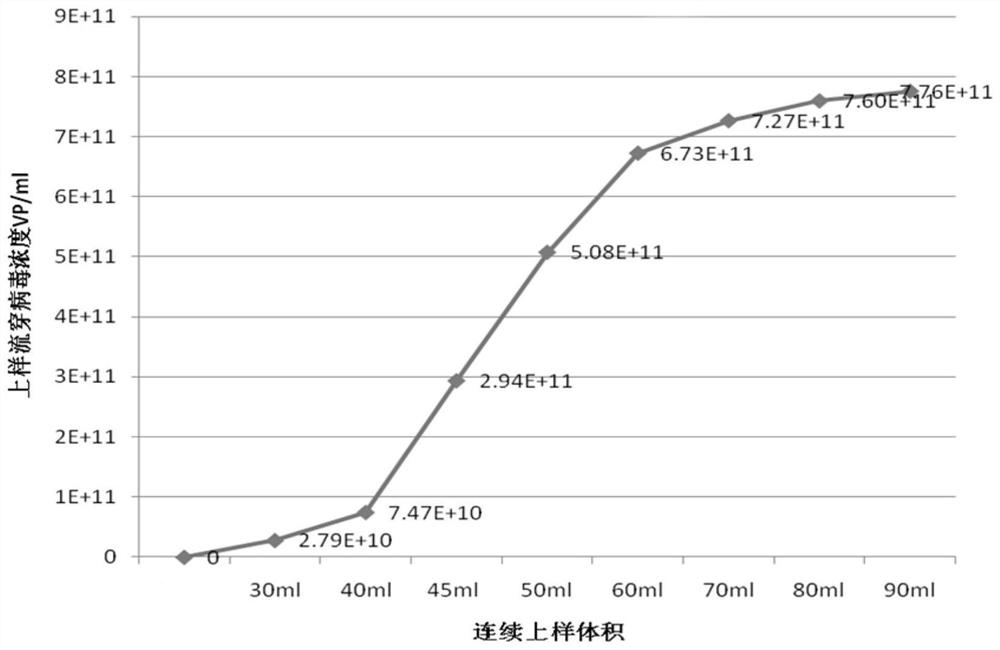
[0037] The method described in step S1 is used for lentivirus packaging and amplification, and the virus liquid is harvested. The method described in step S2 is used to pretreat the virus harvest liquid. In this example, 3ml Q performs membrane chromatography purification method for virus loading optimization, sampling and testing the flow-through liquid at different loading stages, and the specific operation of the purification process refers to step S3. Real-time fluorescent quantitative PCR was used to determine the physical titer of the lentivirus, and the number of copies of the lentivirus detected by the kit was converted into the physical titer VP / ml according to the relationship of VP titer=viral copy number / 2. See the result figure 1 .
[0038] by figure 1 It can be seen that when the sample starts to pass through the membrane column, the virus is completely adsorbed by the ...
Embodiment 2
[0042] Example 2 Comparison of the purification effect of membrane chromatography and traditional ion exchange column chromatography on lentivirus purification.
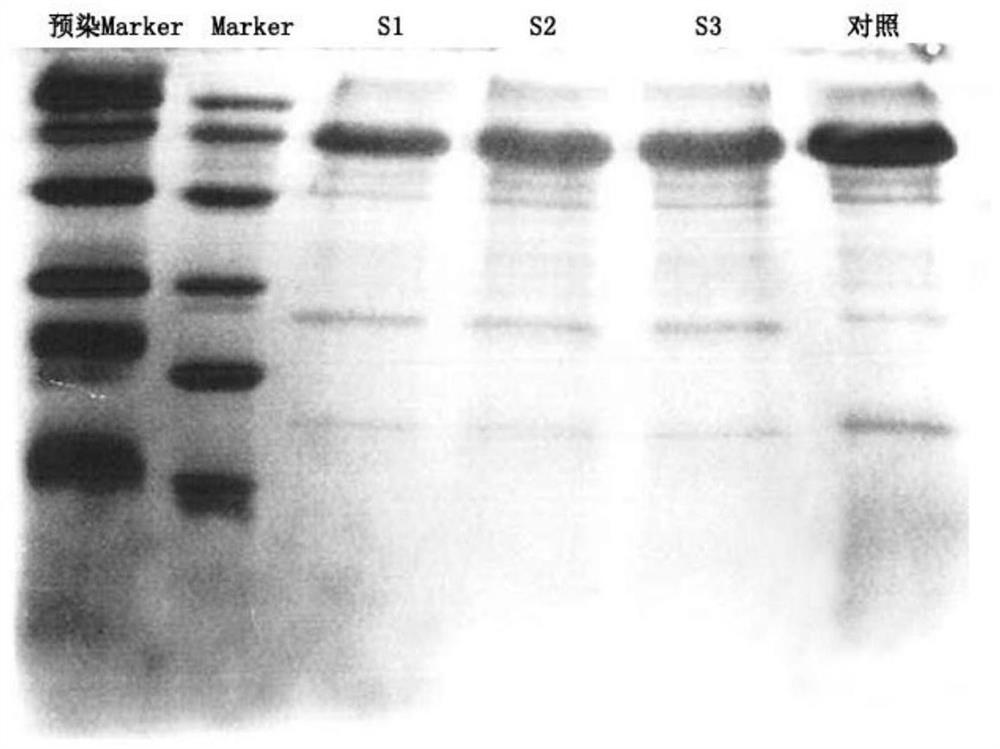
[0043] Host DNA residue is one of the key indicators for evaluating the quality of lentiviral vectors, and DNA is difficult to remove by conventional purification methods. In the past purification process, the method of adding nuclease is often used to degrade and remove the DNA, although certain effects have been achieved However, new exogenous biological reagents are introduced during the processing method, which need to be removed in the subsequent processing process. This not only increases the detection index of the lentiviral vector preparation, but also increases the quality and safety risk of the lentiviral preparation. Purification of lentivirus by membrane chromatography can remove host DNA to a certain extent. In order to evaluate the effect of membrane chromatography on the removal of host DNA and compare th...
Embodiment 3
[0051] Example 3 Scale-up of lentivirus purification process by membrane chromatography.
[0052] Refer to step S1 for lentivirus packaging and amplification, and refer to S2 and S3 for purification. The purified virus samples are sent to the quality department for testing of various indicators. The results are shown in Table 3. It can be seen from the experimental results that the quality of the recombinant lentiviral stock solution obtained by the purification process of the present invention meets the relevant regulations of the 2015 version of the "Chinese Pharmacopoeia" and the "Guiding Principles for Human Gene Therapy Research and Preparation Quality Control Technology".
[0053] Table 3 Test results of lentiviral stock after purification by a certain purification process
[0054] Test items Quality Standard Test results Physical titer vp / ml≥2×10 9
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com