Preparation method of large-size mixed halogen methylamine lead bromine chloride crystal
A large-scale, crystal technology, applied in the field of crystal material preparation, can solve the problem of no large-scale and achieve high-quality results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1CH 3 NH 3 Pb(Br 1-x Cl x ) 3 The preparation of x=0.5 is Br:Cl=1:1
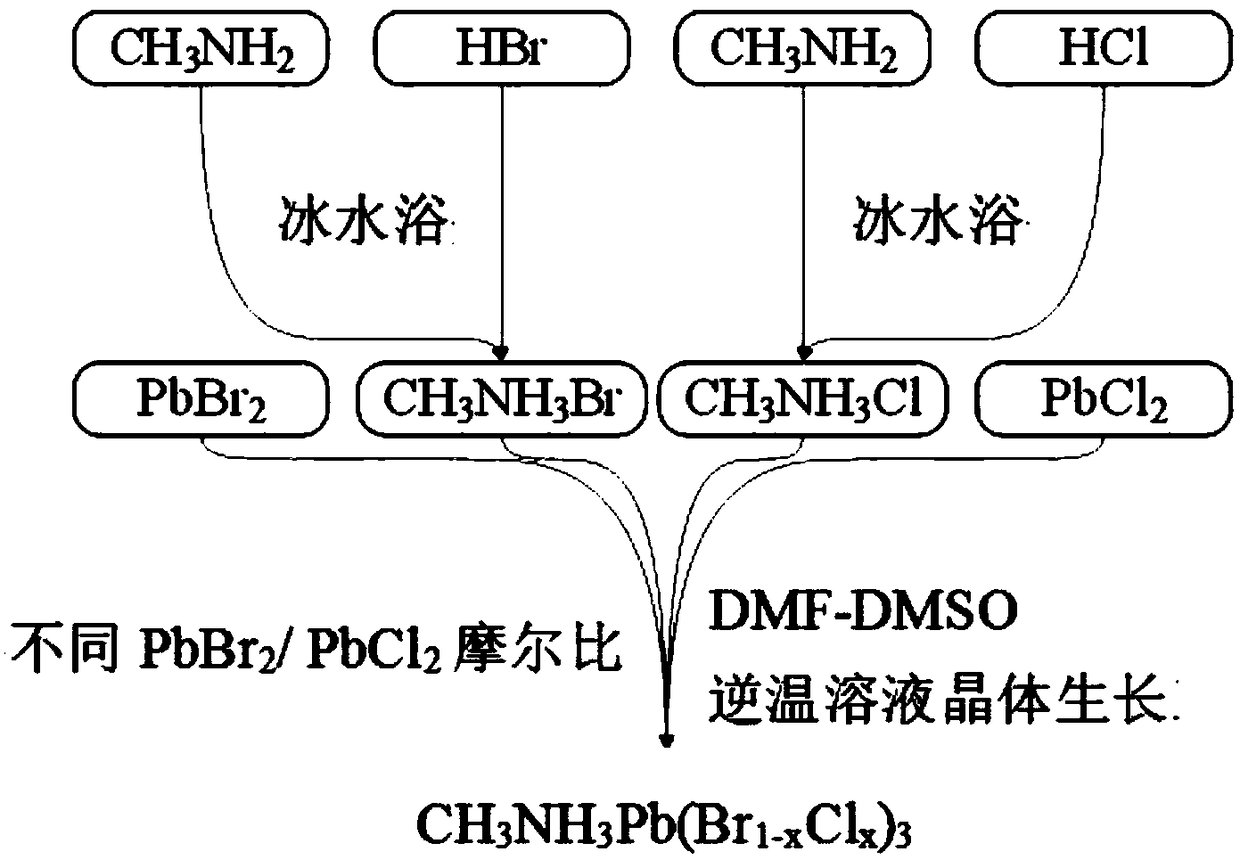
[0028] Comprise following preparation steps (flow process such as figure 1 shown):
[0029] Step S1, CH 3 NH 3 Preparation of Br: Mix monomethylamine aqueous solution (40wt%) and hydrobromic acid aqueous solution (48wt%) according to equimolar solute (0.01mol) in an ice-water bath and stir for 2h, then evaporate to dryness at 100°C to obtain 1.12g white powder thing;
[0030] Step S2, CH 3 NH 3 Preparation of Cl: Mix monomethylamine aqueous solution (40wt%) and hydrochloric acid aqueous solution (37wt%) according to equimolar solute (0.01mol) in an ice-water bath and stir for 2h, then evaporate to dryness at 100°C to obtain 0.67g white powder thing;
[0031] Step S3, preparation of crystallization mother liquor: under normal temperature conditions, PbBr 2 (0.01mol, 3.67g) with CH 3 NH3 Br (0.01mol, 1.12g) and PbCl 2 (0.01mol, 2.78g) with CH 3 NH 3 Cl (0.01mol, 0.67g) was disso...
Embodiment 2-4
[0033] Example 2-4CH 3 NH 3 Pb(Br 1-x Cl x ) 3 preparation of
[0034] By regulating PbBr 2 / PbCl 2 Molar ratio (PbBr 2 with CH 3 NH 3 Br equimolar, PbCl 2 with CH 3 NH 3 Cl equimolar) to prepare crystals with Br:Cl molar ratios of 3:1, 2:1, 1:2, and 1:3, and other conditions were the same as in Example 1.
Embodiment 5
[0035] Embodiment 5 physical parameters are measured
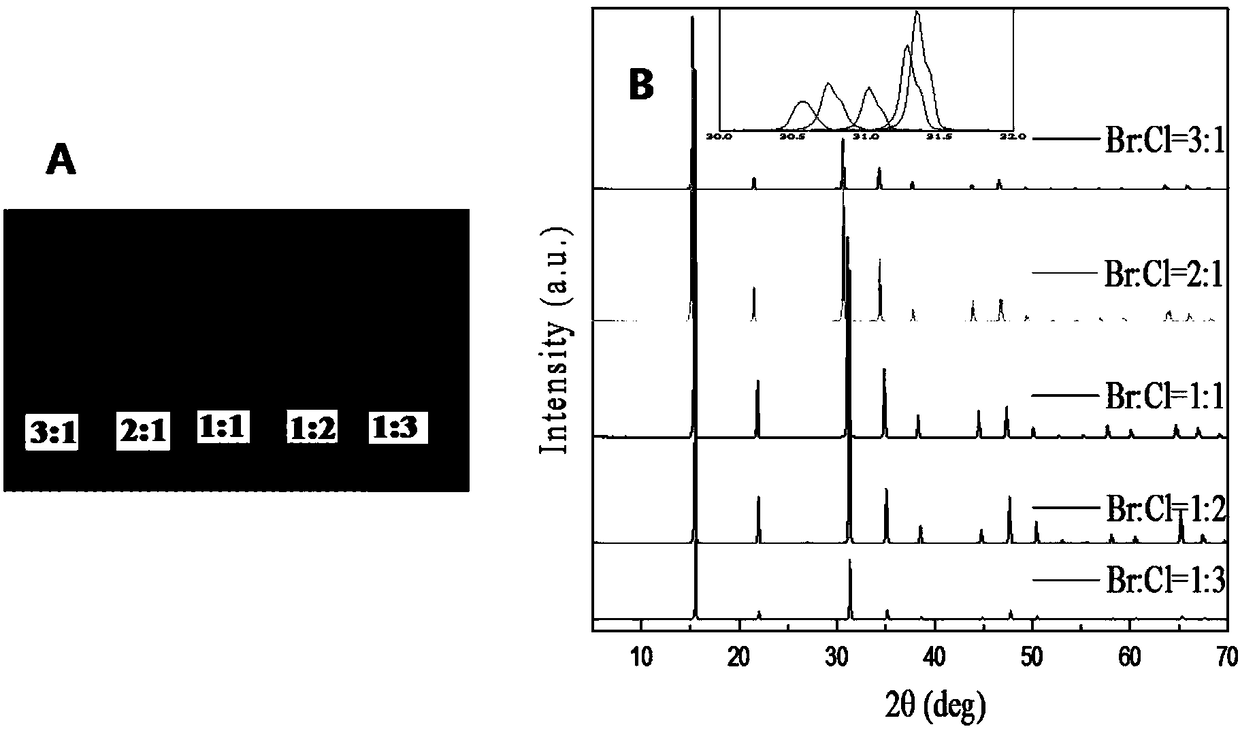
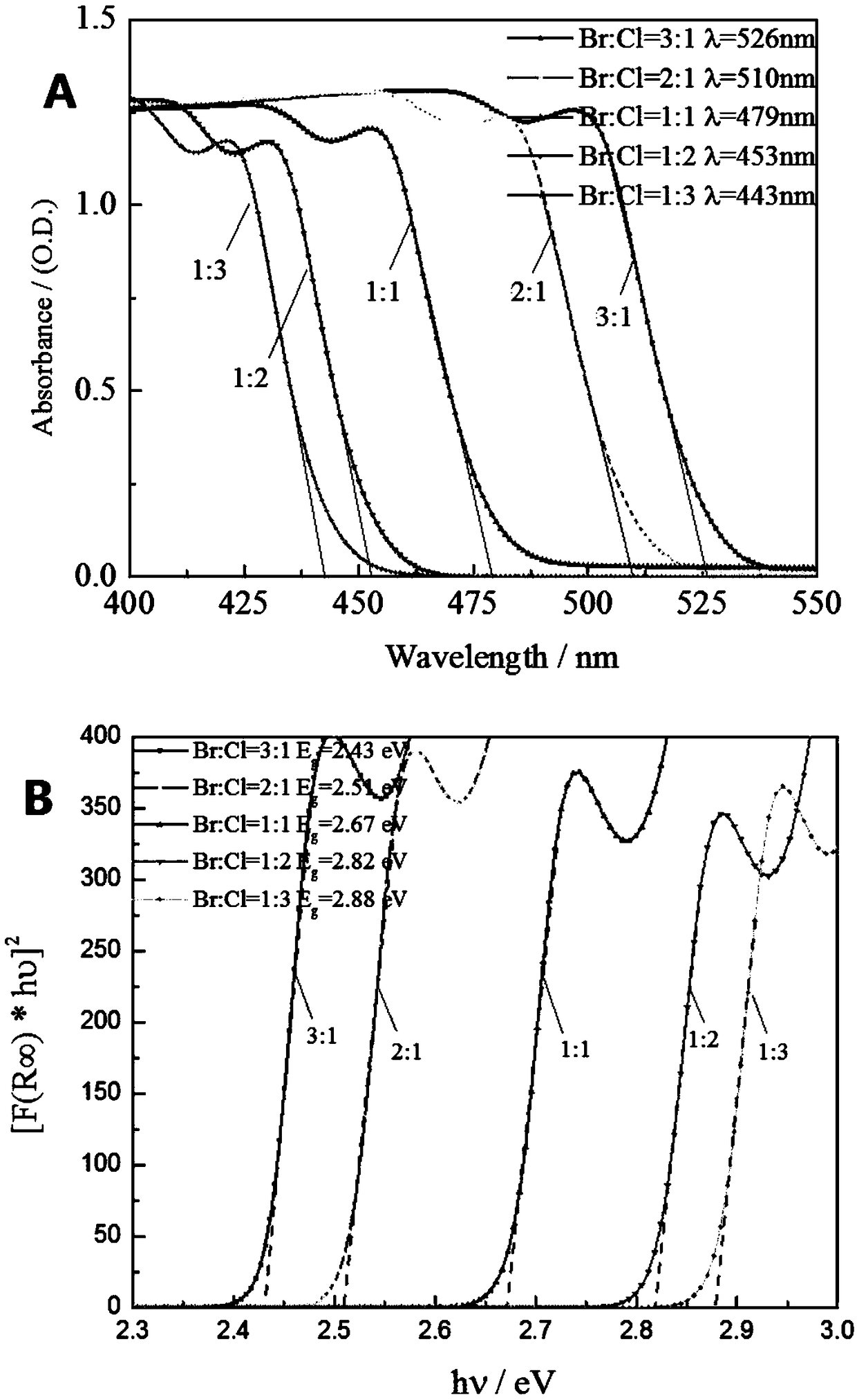
[0036] Measure the CH prepared by embodiment 1-4 respectively 3 NH 3 Pb(Br 1-x Cl x ) 3 The size, XRD, UV-VIS-NIR absorption spectrum and corresponding bandgap spectrum of crystals (Br:Cl molar ratios are 3:1, 2:1, 1:1, 1:2, 1:3, respectively).
[0037] Measurement results such as figure 2 and image 3 As shown, the length and width of the crystals are as high as 6-8mm, and as high as 2-4mm, with ideal spectral properties, adjustable band gap, and high quality.
[0038] The present invention adopts an inversion solution crystal growth process, and by changing the ratio of raw material components, CH with large size, high quality and adjustable band gap can be obtained with different halogen ratios. 3 NH 3 Pb(Br 1-x Cl x ) 3 crystals. The applicant also tried nearly a hundred different solvents, solvent combinations and crystallization processes for the crystallization of large-sized CH 3 NH 3 Pb(Br 1-x Cl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com