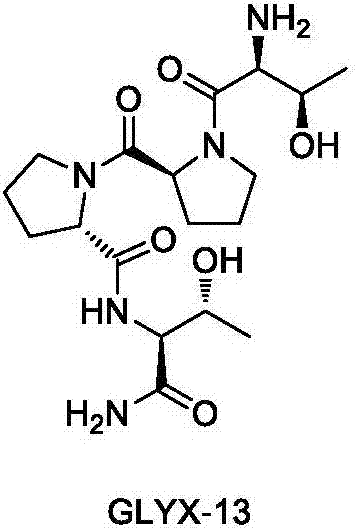
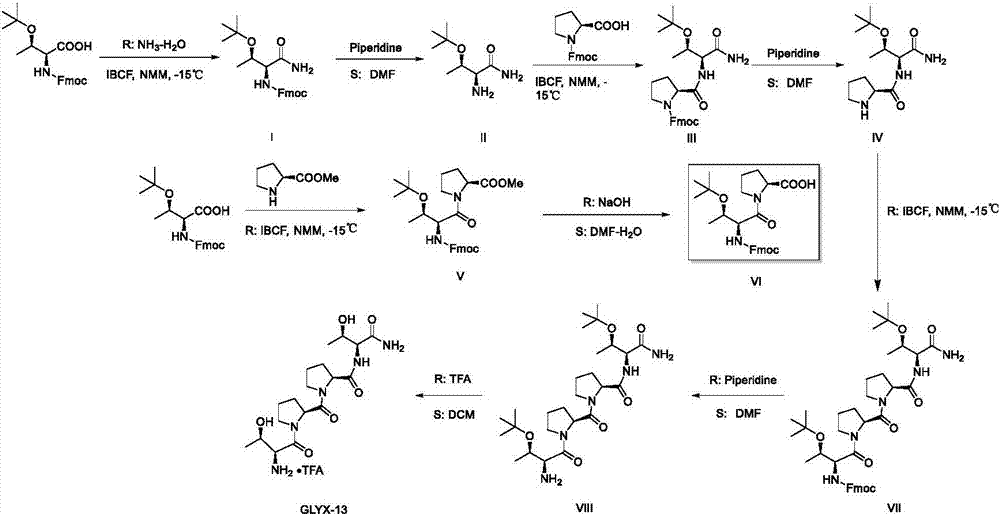
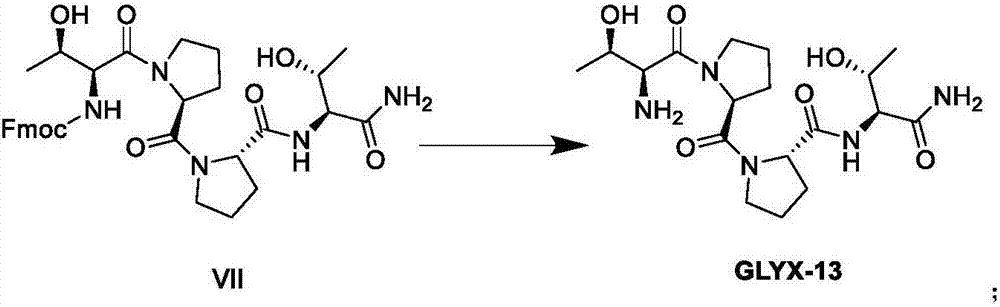
A preparing method of GLYX-13 and a compound used for preparing the GLYX-13
A compound and condensing agent technology, which is applied in the field of compound preparation of GLYX-13, can solve the problems of low synthesis yield, low yield, and difficult removal, etc., and achieve simple post-production treatment, high yield selectivity, and easy removal Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The preparation of embodiment 1 intermediate I
[0053]
[0054]Add N-Cbz-L-threonine amide (2mol, 504g), palladium carbon (50.4g) and anhydrous methanol 3000mL in the autoclave of 5L, mechanical stirring makes N-Cbz-L-threonine amide dissolve, to The reactor was fed with hydrogen to maintain the pressure at 1 MPa, and reacted at room temperature for 12 hours. After the reaction was completed, the palladium carbon was removed by filtration, and the solvent was removed from the filtrate with a rotary evaporator to obtain a crude product of light yellow oil. The crude product was dissolved in 2L of acetone, and a solution of hydrogen chloride in isopropanol was added dropwise in an ice-water bath to precipitate a white solid, which was filtered by suction and vacuum-dried to obtain 289 g of white solid powder, yield: 93%. 1 H NMR (400MHz,D 2 O)δ4.15(p,J=6.1Hz,1H),3.86(d,J=5.2Hz,1H),1.25(d,J=6.5Hz,3H).HRMS(ESI-TOF+)m / z Calcd .forC 4 h 11 N 2 o 2 + [M+H] + :119....
Embodiment 2
[0055] The preparation of embodiment 2 intermediate II
Embodiment 21
[0057]
[0058] Add N-Fmoc-proline (1.5mol, 505g) to a 3L four-necked flask, add 1500mL anhydrous DMF under the condition of nitrogen flow, mechanical stirring makes it dissolve and adds N-methylmorpholine (152g), Make the bottom of the reaction bottle in a low-temperature reactor, slowly add isobutyl chloroformate (205g) dropwise and maintain the temperature of the reaction solution below -15°C, add intermediate I (243g) in solid form after the dropwise addition, and then slowly N-methylmorpholine (152 g) was added dropwise, and the reaction solution was gradually returned to room temperature after the dropwise addition was completed. The reaction solution was transferred to a plastic bucket and purified water (9 L) was added, and 1500 mL of ethyl acetate was added dropwise thereto under the condition of mechanical stirring, a white solid was precipitated, filtered by suction and vacuum-dried to obtain 585 g of a white solid, yield: 89.2%. 1 H NMR (400MHz, DMSO-d 6 )δ7.9...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com