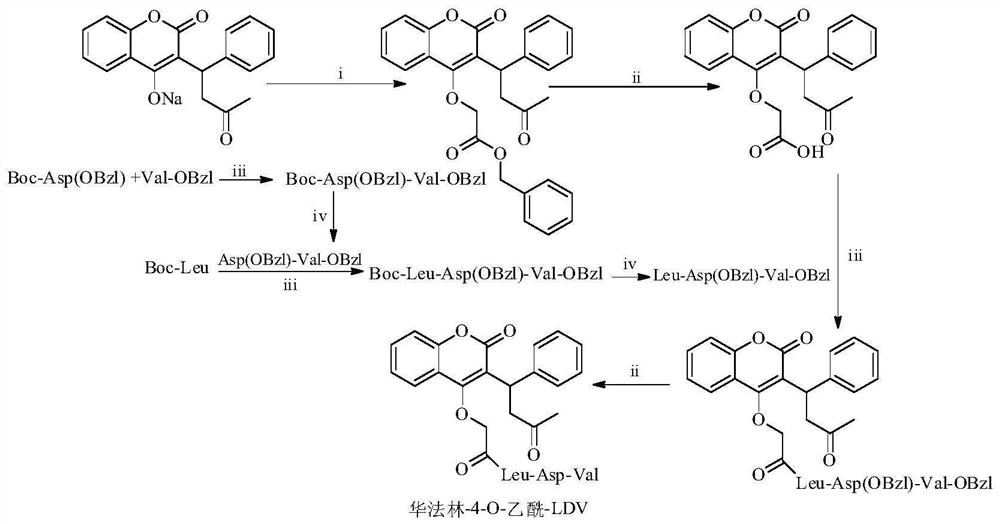
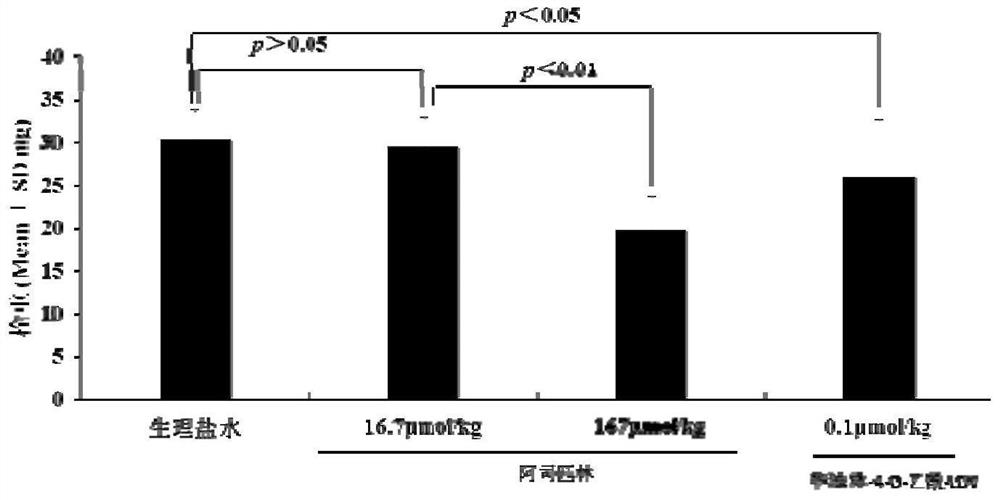
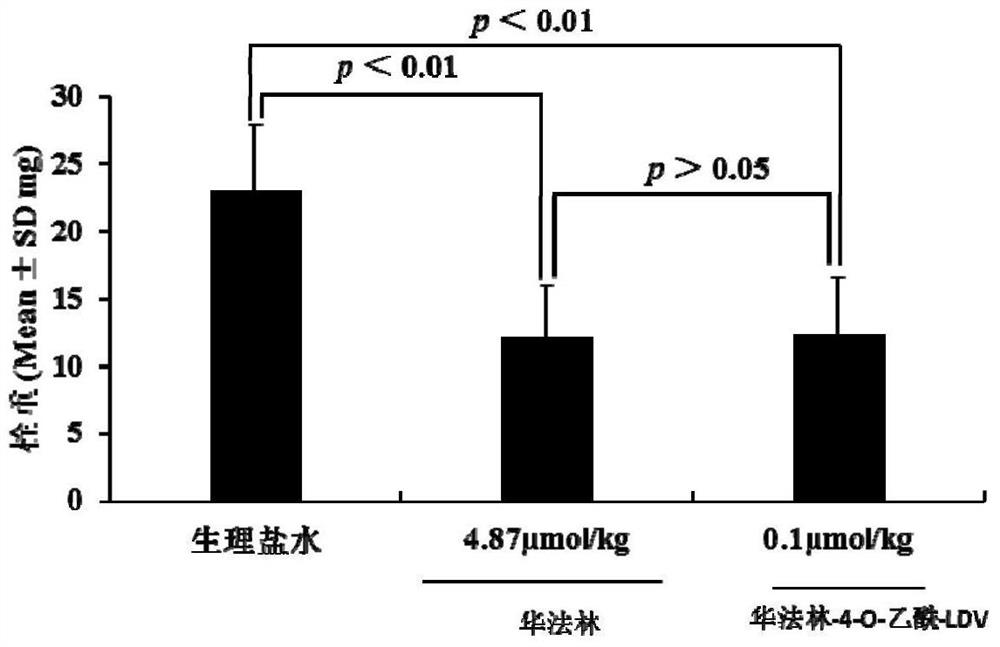
Warfarin-4-o-acetyl-ldv, its synthesis, activity and application
A technology of warfarin,-leu-asp-val, applied in warfarin-4-O-acetyl-LDV, its synthesis, activity and application field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Preparation Wafer Forest-4-O-Benzyl acetate
[0026] 3.31 g (10.00 mmol) of the Waylin Lin was placed in a 100ml of ketchprint, and about 40 ml of acetone was added, and it was not completely dissolved, and the oil bath was added to the gaw of the oil, and 1.73 ml (11.00 mmol) bromine was added. 2-benzyl acetate, continued to replace at 45 ° C oil bath, and the white solid was found to be attached to the wall after about 1 h. After 48 h, thin layer chromatography (TLC, petroleum ether / ethyl acetate = 2: 1) Monitor the reaction process, Hua Fi Lin disappeared, the reaction produced colorless solid was filtered, remove the acetone under reduced pressure, to obtain a light yellow oil Through silica gel column chromatography (petroleum ether / ethyl acetate = 8: 1), 3.02 g (65%) title compound was obtained as a colorless solid. ESI-MS (M / E): 457 [M + H] + ; 1 H-NMR (300MHz, DMSO-D 6 ) δ / ppm = 7.89 (DD, J 1 = 3.0Hz, J 2 = 9.0Hz, 1H), 7.63 (DT, J 1 = 3.0Hz, J 2 = ...
Embodiment 2
[0027] 2.26 g (4.95 mmol) 华 法林 -4-O-acetate benzyl ester is dissolved in 20 mL of methanol, adding 220 mg of palladium carbon (PD / C), stirring to penetrate the air in the water pump, passing into hydrogen, and repeating Three times, stirring at a hydrogen room temperature for 10 h. The TLC was monitored, and PD / C was removed, and the filtrate was removed and the filtrate was concentrated to remove the solvent. The residue was solidified with petroleum ether, and washed with water to give 1.72 g (93%) title compound, which is a colorless solid. ESI-MS (M / E): 367 [M + H] + ; 1 H-NMR (300MHz, DMSO-D 6 : δ / ppm = 12.86 (S, 1H), 7.90 (D, J = 6.0 Hz, 1H), 7.63 (T, J = 6.0 Hz, 1H), 7.43 ~ 7.34 (M, 4H), 7.27 (T, J = 9.0Hz, 2H), 7.17 (T, J = 9.0 Hz, 1H), 4.99 (T, J = 9.0 Hz, 1H), 4.75 (Q, J 1 = 15.0 Hz, J 2 = 30.0Hz, 2H), 3.54 ~ 3.47 (m, 2H), 2.14 (s, 3h).
Embodiment 3
[0028] Example 3 Preparation Boc-ASP (Obzl) -Val-Obzl
[0029]10.21 g (31.61 mmol) of Boc-ASP (Obzl) was added to 500 ml of ketchprint, dissolved with 250 ml of anhydrous tetrahydrofuran, adding 4.18 g (30.97 mmol) HOBT and 7.65g (37.16) under ice bath (0 ° C). Mmol) DCC, activated 30 min. There is a large number of DCU precipitation. 11.73 g (30.94 mmol) TOS · VAL-OBZL was dissolved in 150 ml of anhydrous tetrahydrofuran, and it was added to the reaction solution under an ice bath, and the pH was adjusted with N-methyl morpholine (NMM) to 8 ~ 9, After stirring at room temperature for 6 h, TLC (dichloromethane / methanol = 30: 1) was monitored, and the material point disappeared, filtered off DCU, remove the solvent under reduced pressure, dissolved with 100 ml of ethyl acetate, filter it. DCU, filtrate separately with saturated NA 2 CO 3 Solution (40 ml × 3), saturated NaCl solution (40 ml × 3), saturated kHSO 4 Solution (40 ml × 3), saturated NaCl solution (40 ml × 3), saturated...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com