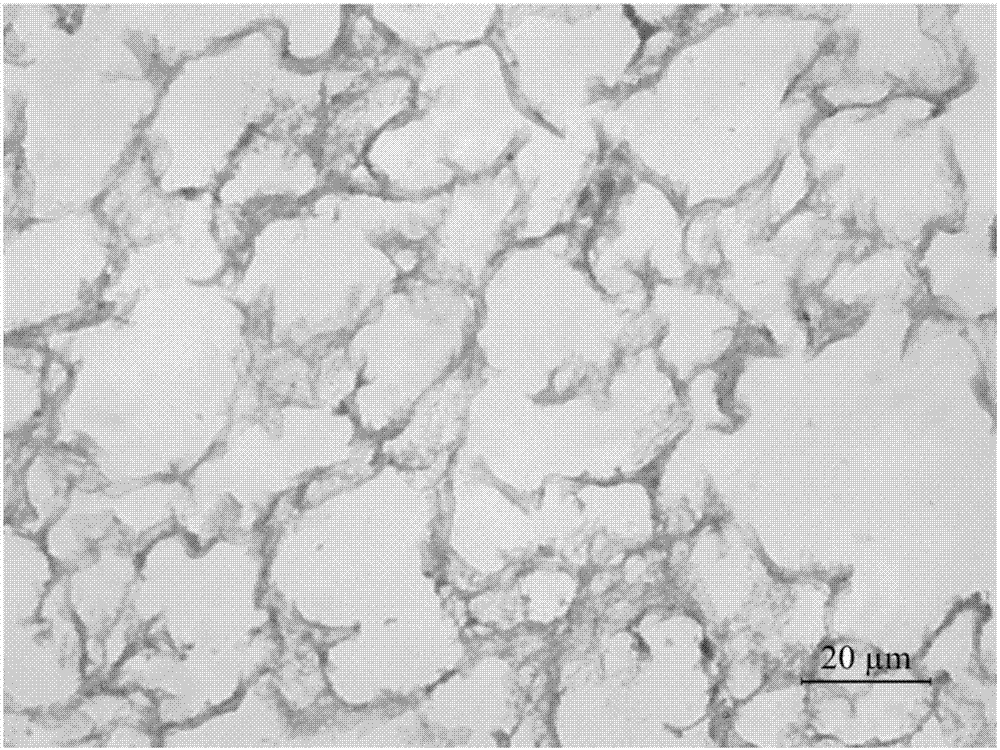
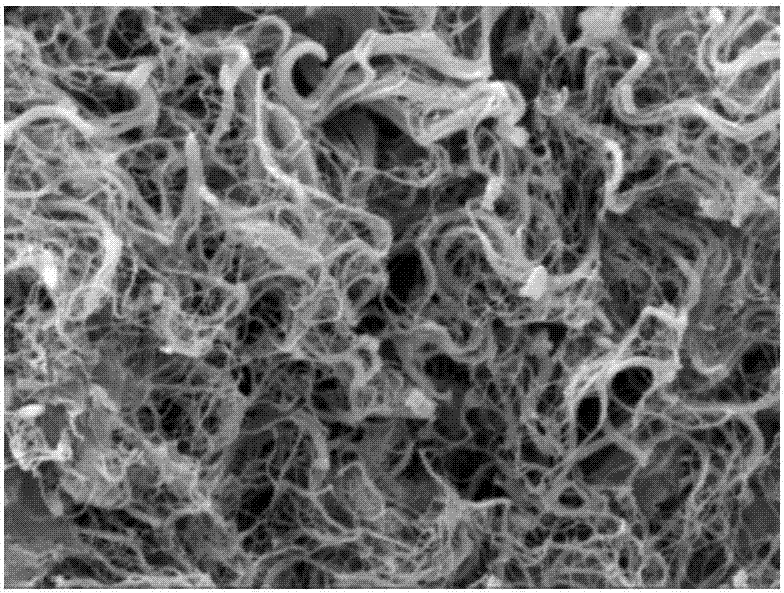
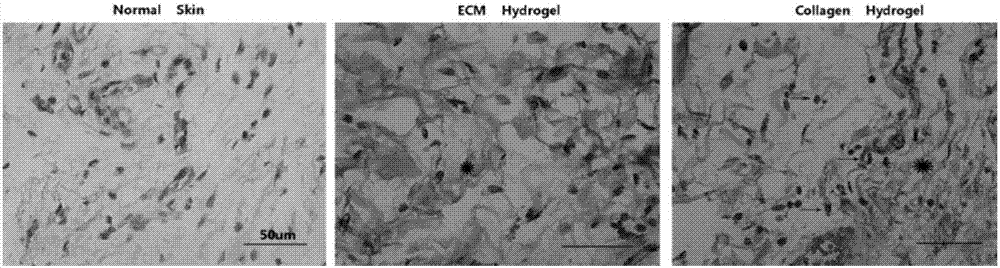
Preparation method and application of lung matrix hydrogel and medicine
A hydrogel and matrix technology, applied in the field of lung matrix hydrogel preparation, can solve the problems of poor selectivity and large side effects, achieve the effects of mild implementation conditions, reduce lung damage, and reduce excessive cell apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A preparation method of lung matrix hydrogel, comprising the following steps:
[0032] The rat decellularized lung tissue is pulverized, and then added into hydrochloric acid-pepsin solution at a ratio of 8mg / ml-12mg / ml for dissolution.
[0033] Add 0.05M-0.15M sodium hydroxide and 8×PBS-12×PBS to the solution after dissolving to adjust to physiological state (at the time of physiological state, pH is about 7.4, hydrogel concentration is about 8mg / ml); Under the condition of 37°C until a jelly-like lung matrix hydrogel is formed.
[0034] Wherein, the preparation method of rat decellularized lung tissue is as follows:
[0035] 1) After the rat is under general anesthesia, the whole body is perfused with heparin, and the complete heart and lung tissue of the rat is taken.
[0036] 2) Insert the trocar into the pulmonary artery through the right ventricle and fix it, set the speed of the peristaltic pump to 4-6ml / min, and pre-fill 0.1% sodium lauryl sulfate to the entir...
Embodiment 2
[0040] On the basis of Example 1, further, after general anesthesia of the rat, the abdominal aorta was intubated and 50 units / mL of heparin was used to perfuse the whole body, and the complete heart and lung tissue of the rat was obtained. Insert the trocar into the pulmonary artery through the right ventricle and fix it. The peristaltic pump adopts a card-type peristaltic pump. The peristaltic pump prefills 0.1% sodium lauryl sulfate to the entire perfusion pipeline at a speed of 5ml / min. Sequentially perfuse with 0.1% sodium lauryl sulfate for 2 hours, deionized water for 15 minutes, 1% Triton X-100 solution for 10 minutes, and double-antibody-added PBS for 72 hours until the tissue is transparent.
Embodiment 3
[0042] On the basis of Example 1, further, the step of comminuting the rat decellularized lung tissue is as follows: after the rat decellularized lung tissue is freeze-dried at a low temperature in a low-temperature freeze-dryer, it is ground into a powder with a pulverizer, and then powdered with ethylene oxide Alkanes for disinfection. Prepare the hydrochloric acid-pepsin solution according to the ratio of 10mg:10ml, add the rat decellularized lung tissue powder into the hydrochloric acid-pepsin solution according to the ratio of 10mg / ml, stir slowly at room temperature for 48 hours until dissolved, and centrifuge at 4°C and 10000rpm / min Remove undissolved substances; adjust to physiological state (PH=7.4) by adding 0.1M sodium hydroxide and 10×PBS, and place at 37° C. until gelatinous lung matrix hydrogel is formed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com