Synthetic method of indol norborneol alkanes and derivative thereof
A technology for norbornane and a synthesis method is applied in the field of synthesis of indolonorbornanes and derivatives thereof, and achieves the effects of simple synthesis method, broad application prospect and wide biological activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
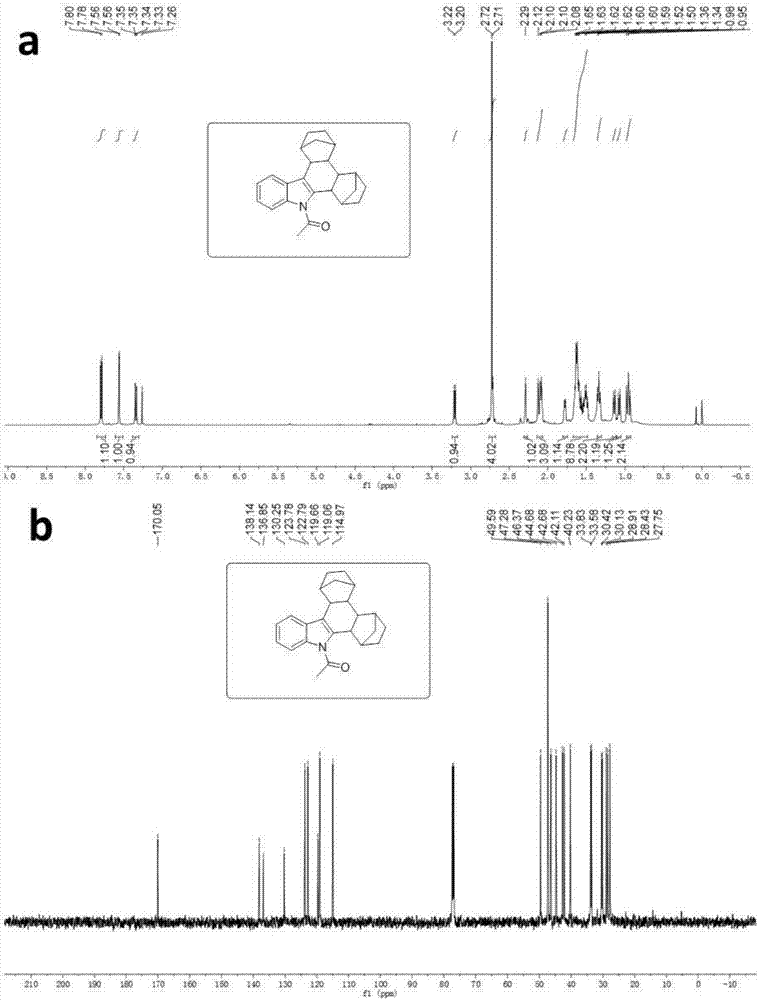
[0032] Synthesis of N-acetylindolonorbornane
[0033] Dissolve indole in methanol solvent and mix evenly for reaction. After ten minutes, add iodine, potassium iodide, and sodium hydroxide, and react at room temperature for 3 hours. After 3 hours, monitor the end of the reaction by TLC. After distilling off the solvent, add acetyl chloride and tetrabutylsulfuric acid. Ammonium hydrogen and sodium hydroxide were dissolved in dichloromethane and mixed uniformly, stirred and reacted at room temperature for 3 hours, then the impurities were removed with a short silica gel column, and the solvent was evaporated to obtain a crude product, which was subjected to column chromatography (ethyl acetate:petroleum ether= 4:96) to obtain 3-iodo-N-acetylindole compound after separation. Dissolve 3-iodo-N-acetylindole compound, palladium acetate, tris(2-furyl)phosphine (TFP), norbornene and cesium carbonate in 1,4-dioxane solvent, mix well and place in React in a thick-walled pressure-resist...
Embodiment 2
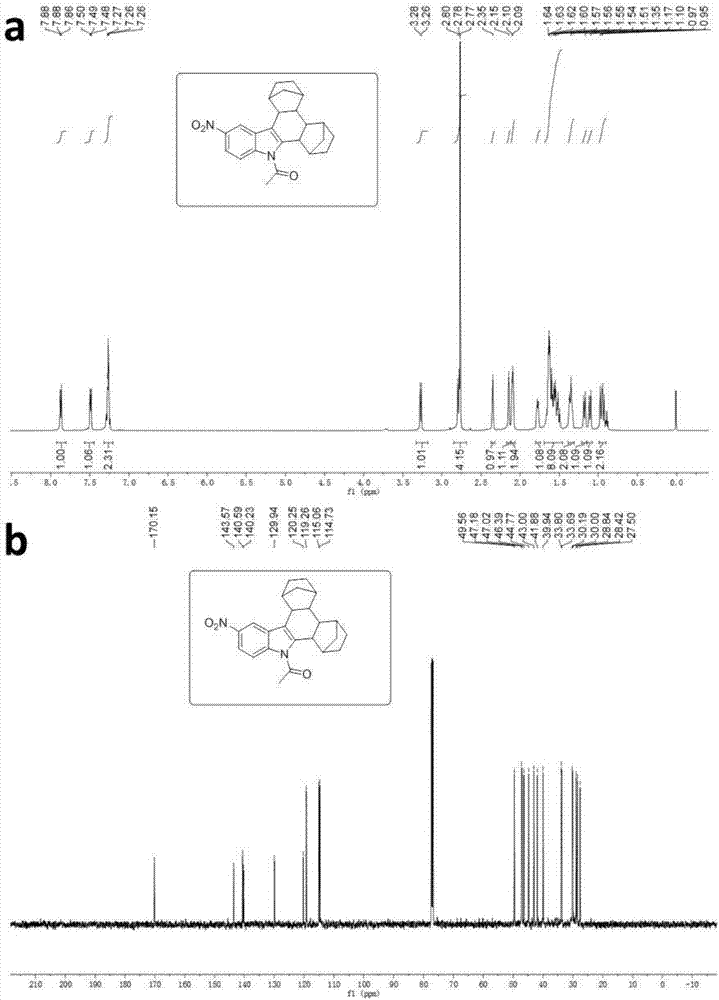
[0035] Synthesis of 5-nitro-N-acetylindolonorbornane
[0036] Dissolve 5-nitroindole in methanol solvent and mix uniformly for reaction. Add iodine element, potassium iodide, and sodium hydroxide after ten minutes, and react at room temperature for 3 hours. After 3 hours, monitor the end of the reaction by TLC. After distilling off the solvent, add acetyl chloride, Tetrabutylammonium bisulfate and sodium hydroxide were dissolved in dichloromethane and mixed uniformly, stirred and reacted at room temperature for 3 hours, then removed impurities with a short silica gel column, evaporated the solvent to obtain a crude product, and the crude product was subjected to column chromatography (ethyl acetate : Petroleum ether=5:95) After separation, the 5-nitro-3-iodo-N-acetylindole compound was obtained. Dissolve 5-nitro-3-iodo-N-acetylindole compound, palladium acetate, tris(2-furyl)phosphine (TFP), norbornene, cesium carbonate in 1,4-dioxane solvent , mixed evenly and reacted in a t...
Embodiment 3
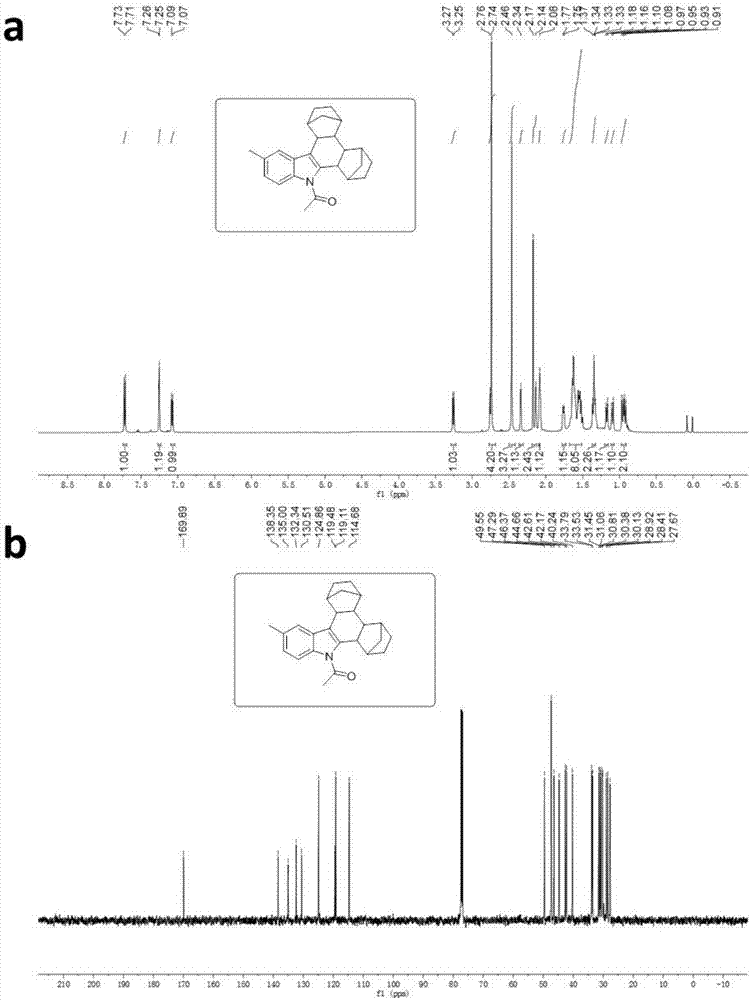
[0038] Synthesis of 5-Methyl-N-acetylindolonorbornane
[0039] Dissolve 5-methylindole in methanol solvent and mix uniformly for reaction. After ten minutes, add iodine, potassium iodide, and sodium hydroxide, and react at room temperature for 1 hour. After 1 hour, monitor the end of the reaction by TLC. After distilling off the solvent, add acetyl chloride, Tetrabutylammonium bisulfate and sodium hydroxide were dissolved in dichloromethane and mixed uniformly, stirred and reacted at room temperature for 24 hours, then removed impurities with a short silica gel column, evaporated the solvent to obtain a crude product, and the crude product was subjected to column chromatography (ethyl acetate : Petroleum ether=5:95) After separation, the 5-methyl-3-iodo-N-acetylindole compound was obtained. Dissolve 5-methyl-3-iodo-N-acetylindole compound, palladium acetate, TFP, norbornene, and cesium carbonate in 1,4-dioxane solvent, mix well and place in a thick-walled pressure-resistant tu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com