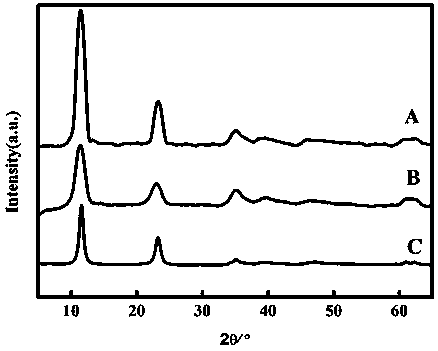
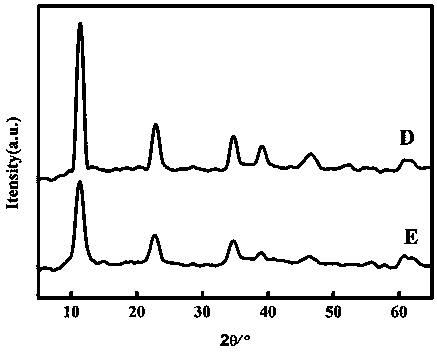
Method for rapidly preparing chlorine ion intercalation cobalt-aluminum hydrotalcite
A technology of chloride ion and hydrotalcite, applied in chemical instruments and methods, cobalt compounds, inorganic chemistry, etc., to achieve the effect of simple and rapid preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Use a pipette to pipette 18.00±0.01 mL of 0.2 mol L -1 sodium acetate solution into a 100 mL volumetric flask, and then add 82.00±0.01 mL 0.2 mol L -1 acetic acid solution, shake it to make it evenly mixed, and make it into 100 mL pH=4.00±0.01 acetic acid-sodium acetate buffer solution.
[0036] (2) Weigh 8.57±0.01 g of cobalt chloride hexahydrate and 4.35±0.01 g of aluminum chloride hexahydrate, mix them into a beaker, add 90±1 mL of deionized water into the beaker, and stir with a magnetic stirrer for 10 min to dissolve it completely, the prepared solution ( c (Co 2+ )=0.40 mol L -1 , c (Al 3+ )=0.20 mol L -1) into a 250 mL hanging bottle infusion set for use.
[0037] (3) Weigh 4.00±0.01 g of sodium hydroxide into a beaker, add a small amount of deionized water into the beaker, and stir with a magnetic stirrer for 10 min to completely dissolve it. Transfer the dissolved solution to a 100 mL volumetric flask to make 1.00mol L -1 sodium hydroxide aqueou...
Embodiment 2
[0042] (1) Pipette 49.00±0.01 mL 0.2 mol L -1 sodium acetate solution into a 100 mL volumetric flask, and then add 51.00±0.01 mL 0.2mol L -1 acetic acid solution, shake it to make it evenly mixed, and make it into 100 mL pH=4.60±0.01 acetic acid-sodium acetate buffer solution.
[0043] (2) Weigh 21.41±0.01 g of cobalt chloride hexahydrate and 7.24±0.01 g of aluminum chloride hexahydrate, mix them into a beaker, add 100±1 mL of deionized water into the beaker, and stir with a magnetic stirrer for 10 min to dissolve it completely, the prepared solution ( c (Co 2+ )=0.90 mol L -1 , c (Al 3+ )=0.30 mol L -1 ) into a 250 mL hanging bottle infusion set for use.
[0044] (3) Weigh 4.00±0.01 g of sodium hydroxide into a beaker, add a small amount of deionized water into the beaker, and stir with a magnetic stirrer for 10 min to completely dissolve it. Transfer the dissolved solution to a 100 mL volumetric flask to make 1.00mol L -1 sodium hydroxide aqueous solution, and th...
Embodiment 3
[0049] (1) Use a pipette to pipette 70.00±0.01 mL of 0.2 mol L -1 sodium acetate solution into a 100 mL volumetric flask, and then add 30.00±0.01 mL 0.2 mol L -1 acetic acid solution, shake it to make it evenly mixed, and make it into 100 mL pH=5.00±0.01 acetic acid-sodium acetate buffer solution.
[0050] (2) Weigh 28.55±0.01 g of cobalt chloride hexahydrate and 7.24±0.01 g of aluminum chloride hexahydrate, mix them into a beaker, add 120±5 mL of deionized water into the beaker, and stir with a magnetic stirrer for 10 min to dissolve it completely, the prepared solution ( c (Co 2+ )=1.00 mol L -1 , c (Al 3+ )=0.25 mol L -1 ) into a 250 mL hanging bottle infusion set for use.
[0051] (3) Weigh 4.00±0.01 g of sodium hydroxide into a beaker, add a small amount of deionized water into the beaker, and stir with a magnetic stirrer for 10 min to completely dissolve it. Transfer the dissolved solution to a 100 mL volumetric flask to make 1.00mol L -1 sodium hydroxide aqu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com