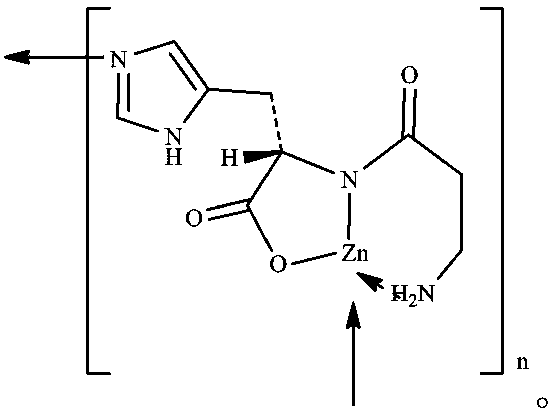
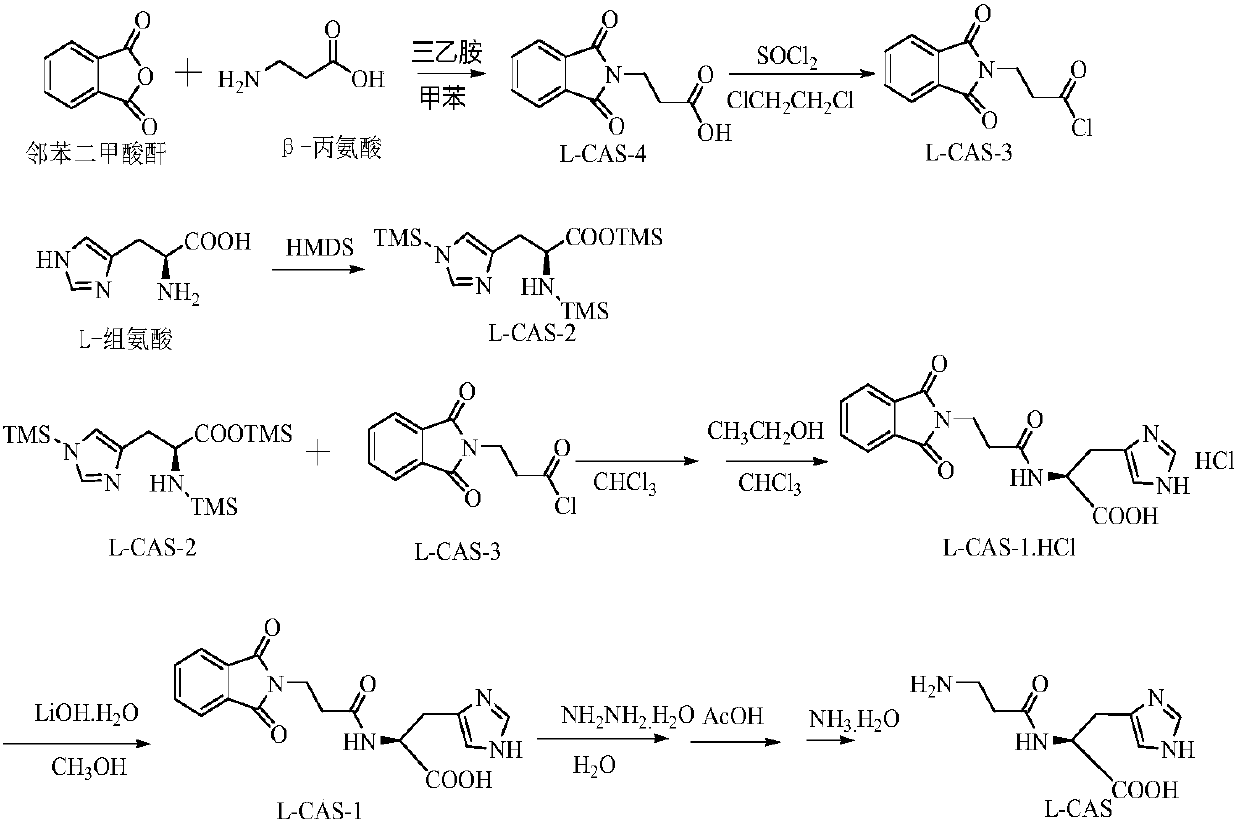
Method for preparing polaprezinc
A technology of polyprezinc and histidine, which is applied in the field of preparation of polyprezinc, can solve the problems of great pressure on environmental protection costs, high production costs, and long time consumption, so as to achieve easy purification and removal of impurities, saving man-hours, filter easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] The preparation method of the polyprezinc of the present embodiment may further comprise the steps:
[0064] Step 1: In a 500mL three-necked flask, 18.4g L-histidine (0.118mol), 1.2g concentrated sulfuric acid (0.01188mol), 66.8g hexamethyldisilazane (0.414mol), 376.8g 1,2 - Dichloroethane was added to the reaction bottle, at normal pressure, 60-65°C, the system was refluxed and kept warm for reaction, the white solid in the system gradually dissolved. After removing the above 1,2-dichloroethane and hexamethyldisilazane, 42.4 g of an orange-yellow residue (liquid) was obtained, with a yield of 96.80%. The residue was uniformly mixed with 57.8 g of dichloromethane to form a solution, namely trimethylsilyl-protected L-histidine in dichloromethane.
[0065] Step 2: Add 24.1g (0.283mol) of 2-cyanoacetic acid, 50.5g (0.425mol) of thionyl chloride, and 152.4g of toluene to the reaction flask in a 1L three-neck flask, heat at 60-62°C, and keep the system under reflux for reac...
Embodiment 2
[0071] The preparation method of the polyprezinc of the present embodiment may further comprise the steps:
[0072] Step 1: In a 500mL three-necked flask, 18.4g L-histidine (0.118mol), 2.3g concentrated sulfuric acid (0.02376mol), 104.7g hexamethyldisilazane (0.649mol), 376.8g 1,2 - Dichloroethane was added to the reaction flask, and at normal pressure and temperature of 60-65°C, the system was refluxed and kept warm for reaction. The white solid in the system gradually dissolved. After 13 hours of reaction, Hexamethyldisilazane and 1,2-dichloroethane were evaporated to obtain 43.0 g of orange-yellow residue (liquid), with a yield of 98.2%. The residue was mixed with 58.6 g of dichloromethane to form a solution, which was a solution of trimethylsilyl-protected L-histidine in dichloromethane.
[0073]Step 2: Add 24.1g (0.283mol) 2-cyanoacetic acid, 68.3g (0.538mol) oxalyl chloride, and 152.4g toluene to a 1L three-necked flask in sequence, heat at 60-62°C, and reflux the syste...
Embodiment 3
[0079] The preparation method of the polyprezinc of the present embodiment may further comprise the steps:
[0080] Step 1: In a 500mL three-necked flask, 18.4g L-histidine (0.118mol), 1.2g concentrated sulfuric acid (0.01188mol), 66.8g hexamethyldisilazane (0.414mol), 376.8g 1,2 - Dichloroethane was added to the reaction bottle, under normal pressure and temperature of 60-65°C, the system was refluxed and kept warm for reaction, the white solid in the system gradually dissolved, and after 16 hours of reaction, it was Hexamethyldisilazane and 1,2-dichloroethane were evaporated to obtain 43.2 g of orange-yellow residue (liquid), with a yield of 98.6%. The residue was uniformly mixed with 58.9 g of dichloromethane to form a solution, namely trimethylsilyl-protected L-histidine in dichloromethane.
[0081] Step 2: In a 1L three-necked flask, add 24.1g (0.283mol) 2-cyanoacetic acid, 84.2g (0.708mol) thionyl chloride, and 152.4g toluene to the reaction flask in sequence, heat at 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com