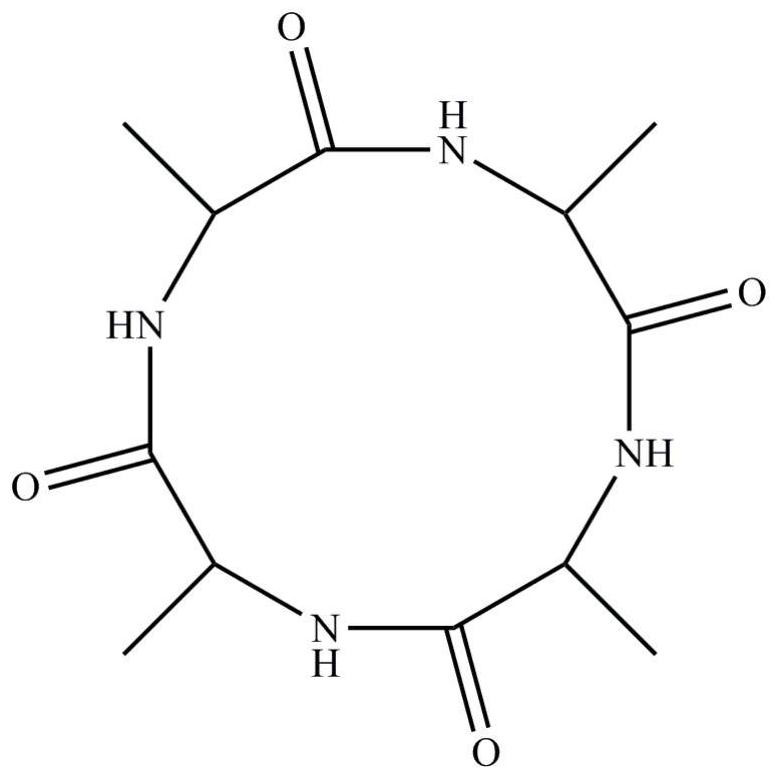
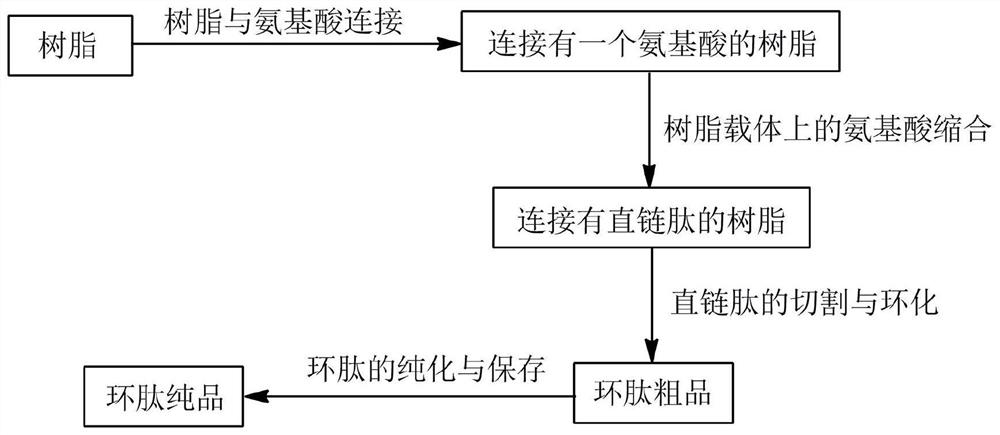
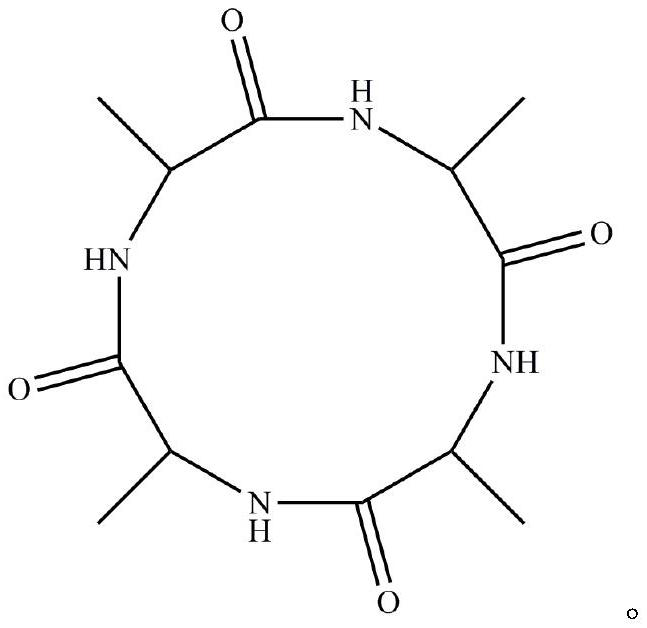
Preparation method of homocyclopeptide cyclo-(ala)4
A technology of cyclic peptide and resin, which is applied in the field of cyclic peptide compound cyclic peptide Cyclo-4 and its synthesis and preparation technology, to achieve good fat solubility and stability in vivo, high synthesis efficiency, good regularity and multi-directional symmetry.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A homocyclic peptide Cyclo-(Ala) 4 preparation methods, including:
[0028]Step 1. Soak 2-chlorotrityl chloride resin with dichloromethane and shake for 30 minutes; remove the solvent after the resin is swollen, and take a three-fold molar excess of alanine with fluorenyl moxycarbonyl protecting group in the resin 1. N,N-diisopropylethylamine is added to the resin in a ten-fold molar excess, then DMF is added to dissolve it, and the reaction is shaken at room temperature for 30 minutes; then methanol is added to incubate for 20 minutes; the reactive sites on the resin are sealed; Wash the resin repeatedly with DMF and methanol solvent and remove the solvent. The first amino acid is connected to the resin; After fully washing with ethanol, add one drop each of 5% ninhydrin ethanol solution, 0.3% ascorbic acid solution, and 60% phenol solution, and heat to 110°C and keep it warm for 5 minutes. If the resin color turns dark blue, then It shows that the amino acid has bee...
Embodiment 2
[0033] A homocyclic peptide Cyclo-(Ala) 4 preparation methods, including:
[0034] Step 1. Soak 2-chlorotrityl chloride resin with dichloromethane and shake for 30 minutes; remove the solvent after the resin is swollen, and take a three-fold molar excess of alanine with fluorenyl moxycarbonyl protecting group in the resin 1. N,N-diisopropylethylamine is added to the resin in a ten-fold molar excess, then DMF is added to dissolve it, and the reaction is shaken at room temperature for 30 minutes; then methanol is added to incubate for 20 minutes; the reactive sites on the resin are sealed; Wash the resin repeatedly with DMF and methanol solvent and remove the solvent. The first amino acid is connected to the resin; After fully washing with ethanol, add one drop each of 5% ninhydrin ethanol solution, 0.3% ascorbic acid solution, and 60% phenol solution, and heat to 105°C for 5 minutes at the same time. If the resin color turns dark blue, then It shows that the amino acid has be...
Embodiment 3
[0039] A homocyclic peptide Cyclo-(Ala) 4 preparation methods, including:
[0040] Step 1. Soak 2-chlorotrityl chloride resin with dichloromethane and shake for 30 minutes; remove the solvent after the resin is swollen, and take a three-fold molar excess of alanine with fluorenyl moxycarbonyl protecting group in the resin 1. N,N-diisopropylethylamine is added to the resin in a ten-fold molar excess, then DMF is added to dissolve it, and the reaction is shaken at room temperature for 30 minutes; then methanol is added to incubate for 20 minutes; the reactive sites on the resin are sealed; Wash the resin repeatedly with DMF and methanol solvent and remove the solvent. The first amino acid is connected to the resin; After fully washing with ethanol, add one drop each of 5% ninhydrin ethanol solution, 0.3% ascorbic acid solution, and 60% phenol solution, and heat to 105°C for 5 minutes at the same time. If the resin color turns dark blue, then It shows that the amino acid has be...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com