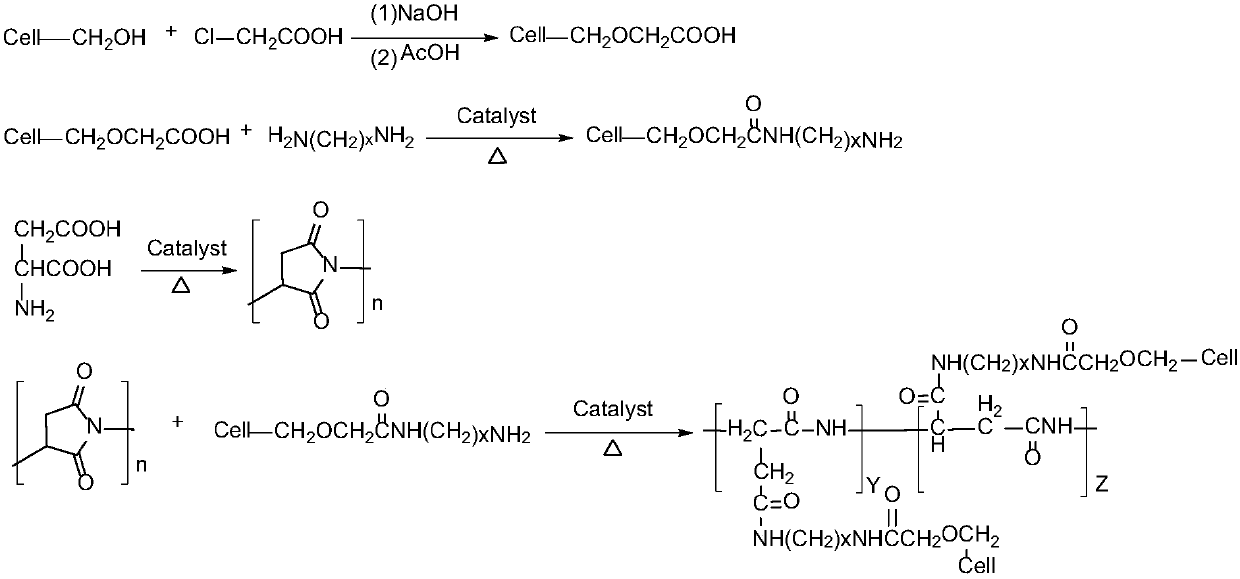
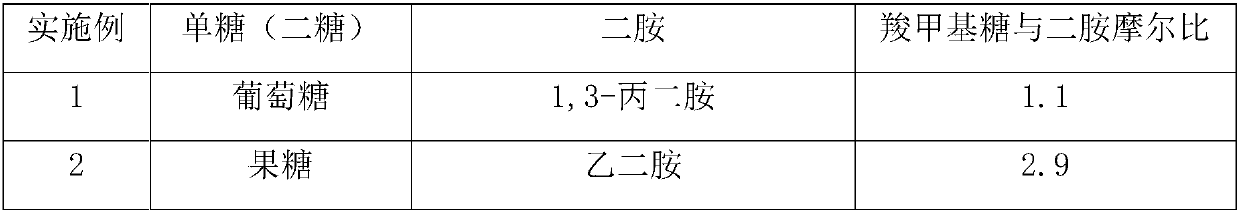
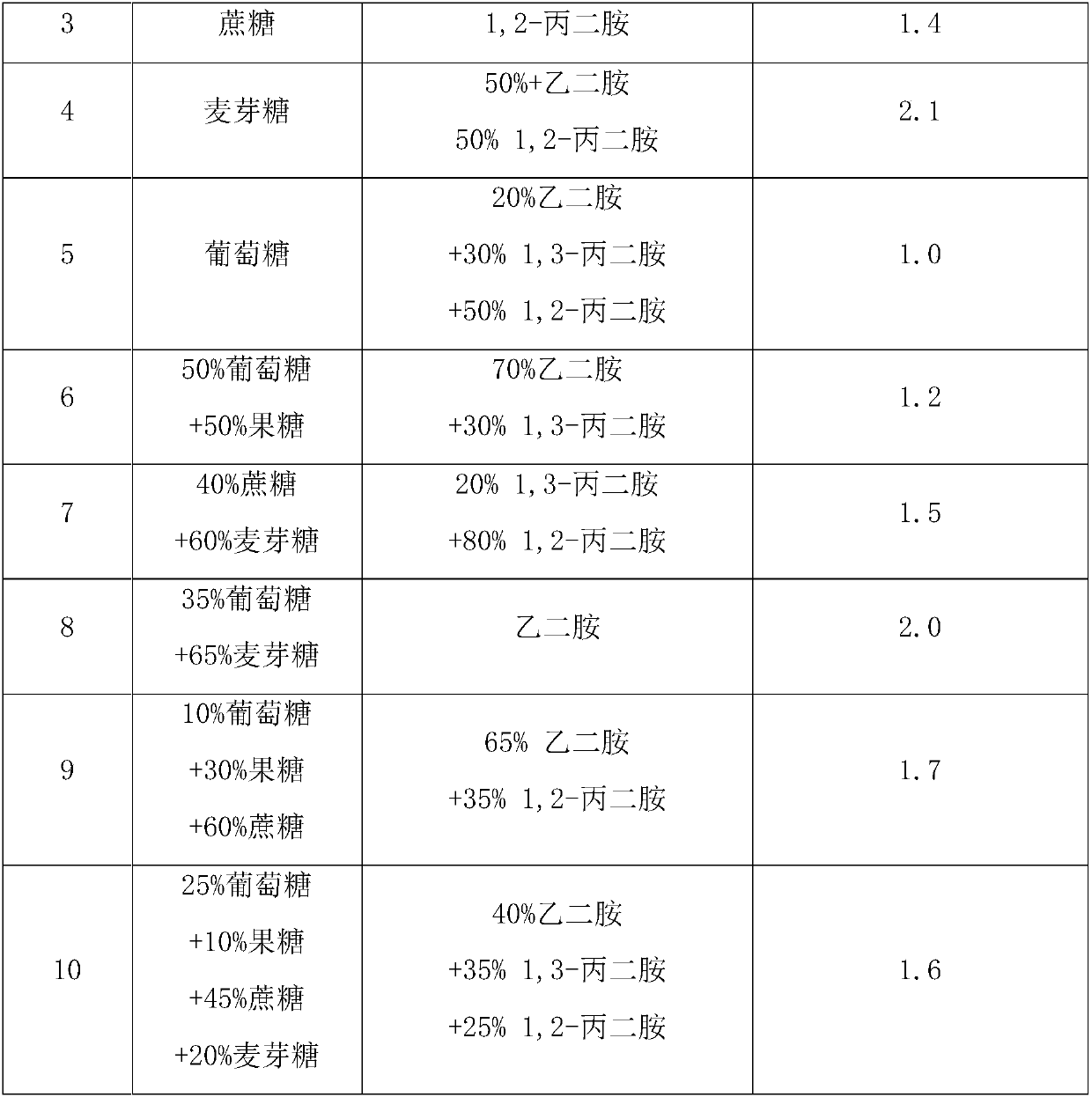
Polyaspartic acid derivative and synthetic method thereof
A polyaspartic acid and synthesis method technology, applied in the field of new polyaspartic acid materials and its synthesis, can solve the problems of high cost, complicated process, uncontrollable molecular weight of polyaspartic acid, etc., and achieve controlled polymerization High degree, simplified process steps, easy to be degraded by microorganisms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
[0041] Example 11: According to Example 2, 100 parts of L-aspartic acid was polymerized for 4 hours in a kneading reactor under the condition of 175°C vacuum degree-20kPa, and then 50 parts of glycosylacetamide was added to carry out a modified ring-opening reaction , After continuing to react for 2h, the polysuccinimide grafted modified product was obtained. Add 3 mol / L aqueous sodium hydroxide solution dropwise to the succinimide modified product obtained by the reaction, hydrolyze for 2.5 hours at 50°C, and obtain polyaspartic acid grafted with glycosyl acetamide after the reaction is completed Derivatives sodium salt solution. The consumption of potassium hydroxide aqueous solution is as the criterion after the pH value of control system is between 8.5~9.5 after finishing the reaction. After subsequent purification steps such as filtration and ultrafiltration, the finally obtained polyaspartic acid derivative saline solution has a mass content greater than or equal to 25%...
Embodiment 12
[0042] Example 12: According to Example 5, 300 parts of L-aspartic acid was polymerized in a kneading reactor at 190°C under the condition of vacuum degree-35kPa for 2 hours, and then 250 parts of glycosylacetamide was added to carry out a modified ring-opening reaction , After continuing to react for 2.5h, a polysuccinimide grafted modified product was obtained. Add 2.5 mol / L aqueous sodium hydroxide solution dropwise to the succinimide modified product obtained by the reaction, hydrolyze at 45°C for 3 hours, and obtain polyaspartic acid grafted with glycosyl acetamide after the reaction is completed Derivatives sodium salt solution. The consumption of potassium hydroxide aqueous solution is as the criterion between 8.5~9.5 with the pH of control system after finishing the reaction. After subsequent purification steps such as filtration and ultrafiltration, the finally obtained polyaspartic acid derivative saline solution has a mass content greater than or equal to 20%, and ...
Embodiment 13
[0043] Example 13: According to Example 7, 150 parts of L-aspartic acid was polymerized for 2.5 hours in a kneading reactor at 205°C under the condition of vacuum degree -50kPa, and then 25 parts of glycosylacetamide was added to carry out modification and ring opening The reaction was continued for 3.5 hours to obtain a modified polysuccinimide graft. Add 5 mol / L aqueous sodium hydroxide solution dropwise to the succinimide modified product obtained by the reaction, hydrolyze at 60°C for 6 hours, and obtain polyaspartic acid derivatives grafted with glycosyl acetamide after the reaction is completed. Sodium saline solution. The consumption of potassium hydroxide aqueous solution is as the criterion after the pH value of control system is between 8.5~9.5 after finishing the reaction. After subsequent purification steps such as filtration and ultrafiltration, the finally obtained polyaspartic acid derivative saline solution has a mass content greater than or equal to 35%, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com