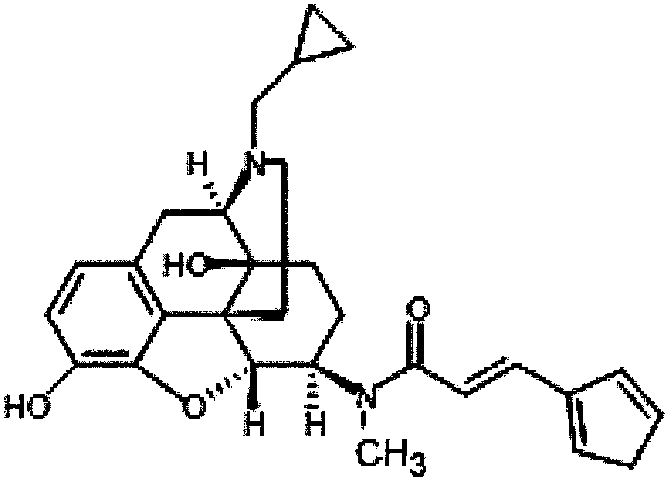
Soft capsule preparation
A technology of soft capsules and capsules, which is applied in the direction of capsule delivery, medical preparations of non-active ingredients, organic active ingredients, etc., which can solve the problems of reduced content stability, complicated drying process, and soft capsule adhesion depressions, etc., to achieve simplification Excellent drying process and content stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The preparation of embodiment 1 soft capsule
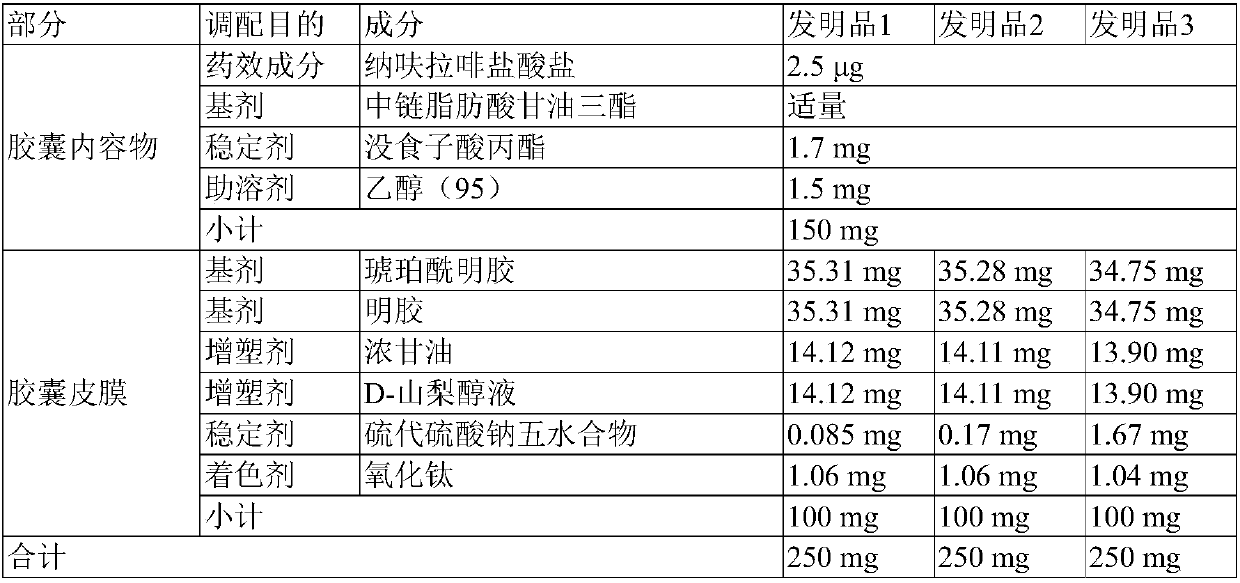
[0045] Dissolve the prescribed amount of nafuraphine hydrochloride and propyl gallate shown in the following table 1 in the prescribed amount of ethanol, and mix it with the prescribed amount of medium-chain fatty acid triglyceride [tri(caprylic acid / capric acid] Acid) glyceride (manufactured by BASF)] and stirred to prepare a capsule filling composition. Stir to disperse the specified amount of gelatin, succinylated gelatin, concentrated glycerin, D-sorbitol solution, sodium thiosulfate and titanium oxide shown in the following Table 1 in an appropriate amount of purified water, and stir at 60°C to dissolve Then, vacuum defoaming was performed to prepare a gelatin film. Soft capsules were prepared using the above-mentioned capsule filling composition and gelatin film by punching using a rotary automatic soft capsule molding machine.
[0046] In addition, 2.5 μg of REMITCH capsules [2.5 μg of nalfurine hydrochloride (manu...
Embodiment 2
[0053] Embodiment 2 stability test
[0054] The soft capsule prepared in Example 1 was put into a glass bottle, and stored at 80° C. for 1 week in a fastened state. Utilize HPLC (high performance liquid chromatography, high-performance liquid chromatography) method to measure the content and decomposition product of nafuraphine hydrochloride in each soft capsule after storage time ends. The measurement results are shown in Tables 3 and 4.
[0055] [table 3]
[0056] Residual rate of nafurphine hydrochloride in soft capsules
[0057]
[0058] [Table 4]
[0059] Amount of decomposed substances in soft capsules
[0060]
[0061] According to Table 3, it can be clearly seen that the product of the present invention has a higher residual rate of nafuraphine hydrochloride than the comparative product, showing remarkable content stability. Also, as is clear from Table 4, the product of the present invention did not increase the 10α-OH form seen in the comparative product, ...
Embodiment 3
[0062] The preparation of embodiment 3 soft capsules
[0063] Soft capsules were prepared in the same manner as in Example 1 in the prescribed amounts shown in Table 5 below.
[0064] In addition, 2.5 μg of REMITCH capsules [2.5 μg of nalfuraphine hydrochloride (manufactured by Toray Co., Ltd.) prepared in one capsule) was used as a comparative product in the same manner as in Example 1.
[0065] [table 5]
[0066] Composition of the invention
[0067]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com