Asiatic acid-chitosan-deoxycholic acid (AA-CS-DCA) micelles and preparation method thereof
A technology of deoxycholic acid and asiatic acid, which can be applied in drug combinations, pharmaceutical formulas, medical preparations of non-active ingredients, etc., and can solve the problems that there is no patent application for asiatic acid micelles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: the synthesis (50KDa) of chitosan deoxycholic acid graft
[0024] Weigh 0.9g of deoxycholic acid and 1.325g of carbodiimide, dissolve them in 50ml of organic solvent (ethanol:acetone=3:7), and activate at 60°C for 1h under magnetic stirring. 1.68g of chitosan with a molecular weight of 50KDa (degree of deacetylation 79.3%) was added to 100ml of deionized water and stirred to dissolve, the above organic solution was added to the aqueous solution, and stirred at 60°C for 8h. The reaction solution was placed in a dialysis bag, dialyzed with deionized water for 24 hours, and the dialysate was freeze-dried to obtain chitosan deoxycholic acid graft. The grafting rate of the synthesized CS-DCA measured by linear potentiometric titration is (6.83±0.10)%; CS-DCA can form self-assembled micelles in water, and its critical micelle concentration is 0.074mg / mL measured by pyrene fluorescence method, The particle size is (34.2±4.3) nm, and the Zeta potential is (43.7±1...
Embodiment 2
[0025] Embodiment 2: the synthesis (126KDa) of chitosan deoxycholic acid graft
[0026] Weigh 0.9g of deoxycholic acid and 1.325g of carbodiimide, dissolve them in 50ml of organic solvent (ethanol:acetone=3:7), and activate at 60°C for 1h under magnetic stirring conditions. Add 1.54 g of chitosan with a molecular weight of 126 KDa (degree of deacetylation: 89.1%), add 100 ml of deionized water and stir to dissolve, add the above organic solution into the aqueous solution, and react with stirring at 60° C. for 8 hours. The reaction solution was placed in a dialysis bag, dialyzed with deionized water for 24 hours, and the dialysate was freeze-dried to obtain chitosan deoxycholic acid graft. The grafting rate of the synthesized CS-DCA measured by linear potentiometric titration is (10.05 ± 0.10)%; CS-DCA can form self-assembled micelles in water, and its critical micelle concentration is 0.158mg / mL measured by pyrene fluorescence method, The particle size is (54.2±2.7) nm, and t...
Embodiment 3
[0027] Embodiment 3: Confirmation of structure of chitosan deoxycholic acid graft
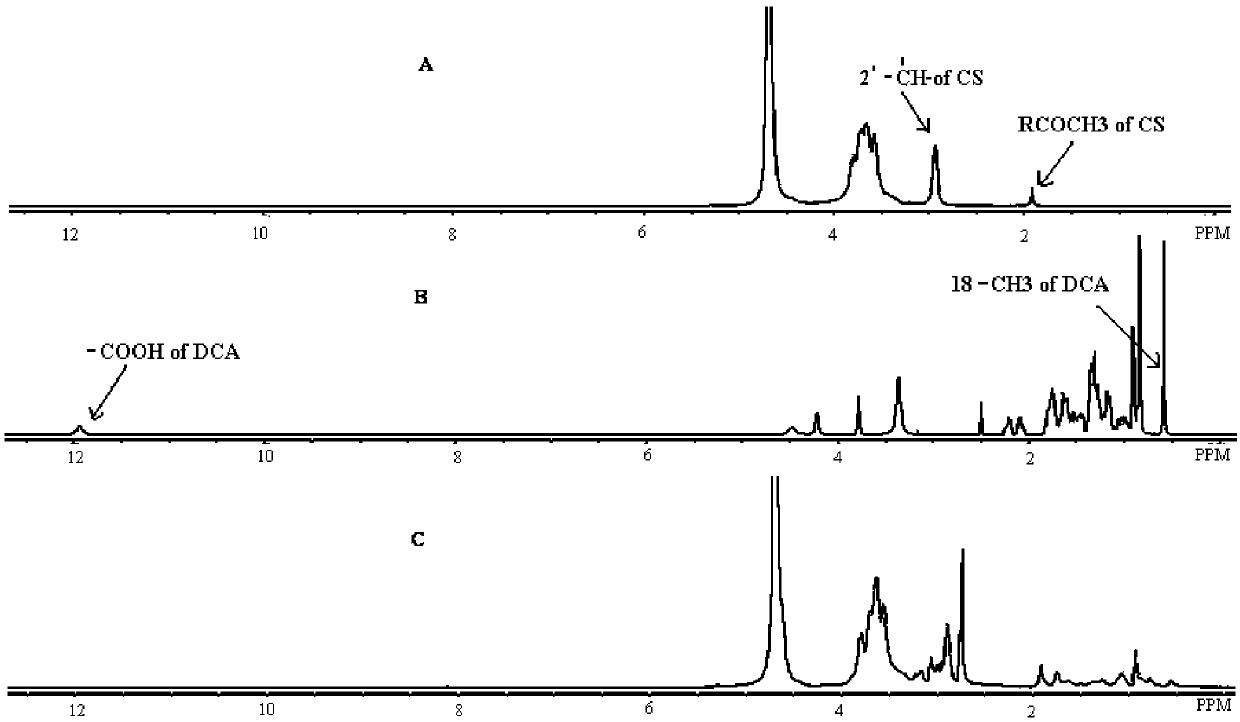
[0028] Under the action of the catalyst, the primary amino group on the chitosan monomer can be used as an active group for chemical grafting reaction, and some functional groups are introduced to obtain a chitosan graft with ideal performance. Considering that DCA is a carboxyl-containing endogenous substance, the physicochemical properties of CS can be significantly improved by grafting DCA onto CS. use 1 The structure of CS-DCA obtained in Example 1 was analyzed by H-NMR.
[0029] The result is as figure 1 As shown, A, B, C are CS, DCA and CS-DCA respectively 1 In the H-NMR spectrum, the peak with a chemical shift of about 12ppm in B is the characteristic peak of DA: the proton peak of -COOH, and this peak is not seen in C (product CS-DCA) and A (CS); C (CS -DCA) appeared in the characteristic peak of DCA (18-CH 3 proton peak), the chemical shift is about 0.6ppm. The above results indi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com