Method for synthesizing fenticonazole nitrate
A kind of technology of fenticonazole nitrate and fenfen nitrate, applied in directions such as organic chemistry, can solve problems such as being unsuitable for industrialized production, high chemical reaction activity, expensive solvent and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
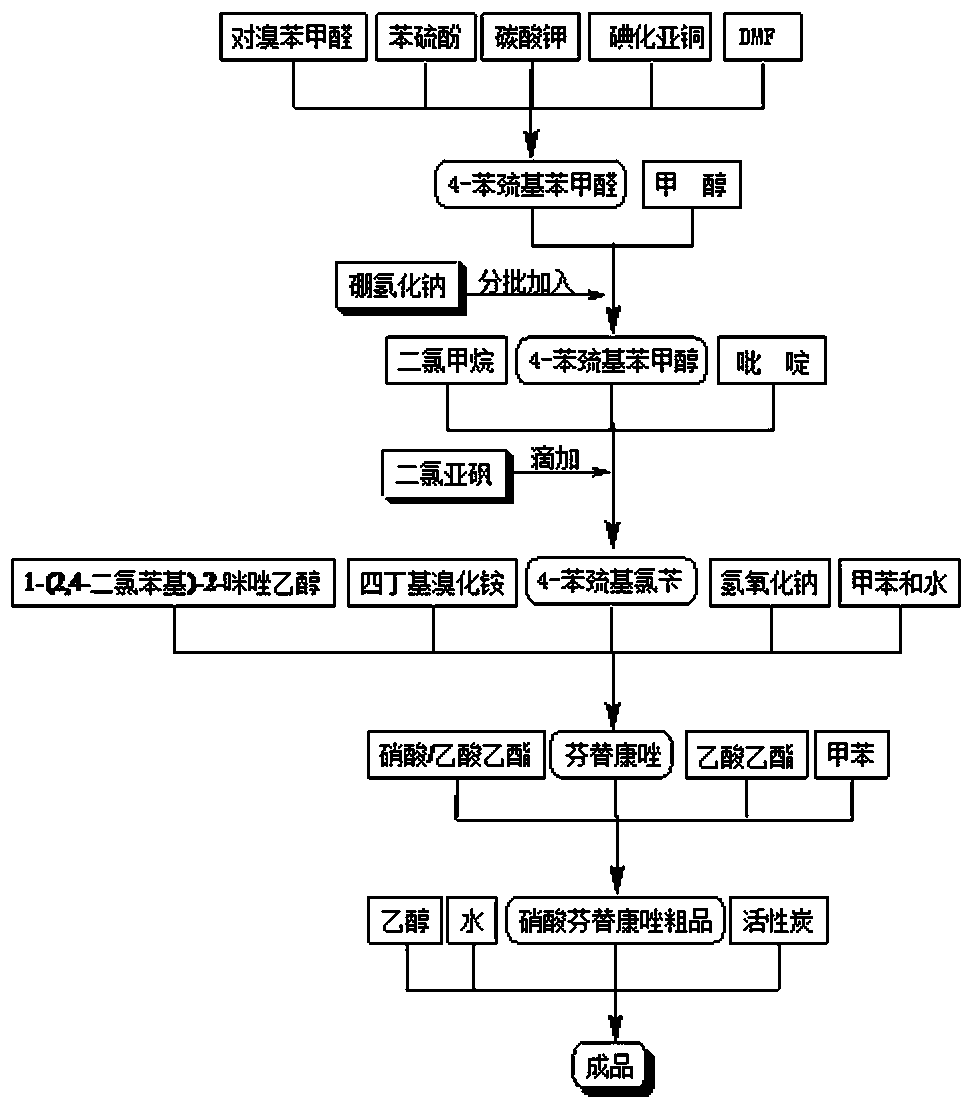
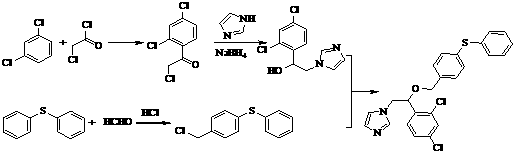
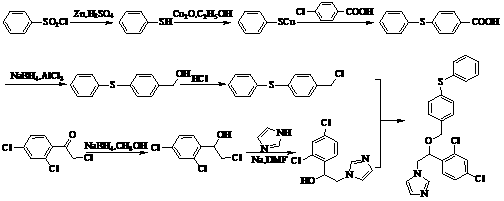
[0031] A method for synthesizing fenticonazole nitrate, which adopts a phase transfer catalysis method, and carries out condensation reaction in the presence of sodium hydroxide, the reagents used are cheap and easy to obtain, the synthesis cost is greatly reduced, the operation is simple and convenient, and there is no special requirement for equipment. It is more suitable for large-scale production, so it is initially planned to adopt this process, so the synthetic process of finally selected fenticonazole nitrate is shown in figure 1 , Synthetic fenticonazole nitrate laboratory test feeding and output situation are shown in Table 1:
[0032] Table 1
[0033]
Embodiment 2
[0035] Intermediate Ⅰ: Synthesis of 4-phenylmercaptobenzaldehyde
[0036] Feed according to the molar ratio of p-bromobenzaldehyde: thiophenol: cuprous iodide = 1:1.15:0.025, stir in DMF and heat up to reflux, TLC detection (n-hexane: ethyl acetate = 10:1) for about 8 hours After the reaction, filter, and concentrate the filtrate to dryness under reduced pressure (pressure 1720Pa, temperature 85°C) to obtain a viscous residue, add a certain amount of dichloromethane, wash with water three times, dry over anhydrous magnesium sulfate, filter, and concentrate the filtrate under normal pressure To dryness, the residue was dissolved in a mixed solvent of n-hexane: ethyl acetate = 1:1, frozen (0°C) for crystallization, filtered, and the filter cake was dried at 25°C under normal pressure to obtain a light yellow crystalline powder.
[0037] Considering that the optimization process should speed up the reaction rate and increase the yield, the influence of the main feed ratio (p-brom...
Embodiment 3
[0043] Intermediate Ⅱ: Synthesis of 4-Phenylmercaptobenzyl Alcohol
[0044]Put 4-phenylmercaptobenzaldehyde and methanol into the reaction flask, add sodium borohydride in batches under stirring, exotherm violently, and naturally raise the temperature to 55°C, TLC detection (n-hexane:ethyl acetate=10:1) to At the end of the reaction, the reaction was about 1 hour. Add hydrochloric acid dropwise to adjust pH=6, filter, and concentrate the filtrate to dryness under reduced pressure (pressure 1330Pa, temperature 45°C), recover methanol to obtain a viscous residue, add appropriate amount of dichloromethane, wash with water until neutral, anhydrous magnesium sulfate Dry, filter, and concentrate the filtrate under reduced pressure (pressure 1720Pa, temperature 25°C), recover dichloromethane, and cool to room temperature to obtain a yellowish solid.
[0045] Considering the optimization process, it is necessary to control the reaction rate and improve the product quality. At the sam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com