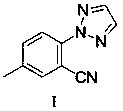
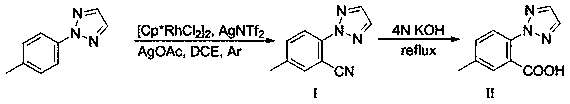
Method for synthesizing 5-methyl-2-(2H-1,2,3-triazole) benzoic acid
A synthesis method and technology of benzoic acid are applied in the field of synthesis of 5-methyl-2-benzoic acid, which can solve the problems of low yield, unfavorable industrial production, difficult separation of by-products, expensive starting materials, etc., and achieve low price, The effect of overcoming impurities that are not easy to separate and good economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
[0036] Under the protection of argon, add 2-(4-methylphenyl)-2 H -[1,2,3]-Triazole (0.2 mmol), N-cyano-N-phenyl-p-toluenesulfonamide (NCTS) (0.4 mmol), catalyst dichloride (pentamethylcyclopentadienyl) Rhodium (III) dimer ([Cp * RhCl 2 ] 2 (0.01 mmol), the oxidant bistrifluoromethanesulfonate silver imide (AgNTf 2 ) (0.06 mmol), additive sodium acetate (NaOAC) (0.2 mmol), 1,2 dichloroethane (DCE) 2 ml, argon gas exchange three times, and react at 150 ℃ for 48h. After the reaction of the raw materials was detected by TLC, the reaction solution was cooled to room temperature, added with water and dichloromethane for extraction, and the organic phase was retained, washed with saturated brine, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and separated on a silica gel column to obtain the product Ⅰ , namely 5-methyl-2-(2 H -1,2,3-triazole) benzonitrile, the conversion rate is 95%, and the yield is 81%.
[0037] White solid, mp 84-86 ℃....
Embodiment 2
[0041]Under the protection of argon, add 2-(4-methylphenyl)-2 H -[1,2,3]-Triazole 0.2 mmol, N-cyano-N-phenyl-p-toluenesulfonamide (NCTS) 0.4 mmol, catalyst dichloro(pentamethylcyclopentadienyl)rhodium(III ) dimer ([Cp * RhCl 2 ] 2 )0.01 mmol, oxidant silver sulfate (Ag 2 SO 4 ) 0.06 mmol, additive silver acetate (AgOAC) 0.2 mmol, 1,2 dichloroethane (DCE) 2 ml, argon gas exchanged three times, and reacted at 150 ℃ for 48 h. After the reaction of the raw materials was detected by TLC, the reaction solution was cooled to room temperature, added with water and dichloromethane for extraction, and the organic phase was retained. The organic phase was dried with saturated saline and anhydrous sodium sulfate, concentrated under reduced pressure, and separated on a silica gel column to obtain the product I. That is, 5-methyl-2-(2 H -1,2,3-triazole) benzonitrile, the conversion rate is 87%, and the yield is 77%.
[0042] Compound I was added to 4 moles per liter of potassium hydr...
Embodiment 3
[0044] Under the protection of argon, add 2-(4-methylphenyl)-2H-[1,2,3]-triazole 0.2mmol, N-cyano-N-phenyl-p-toluenesulfonamide into a 15ml sealed tube (NCTS) 0.4mmol, catalyst dichloro(pentamethylcyclopentadienyl) rhodium (III) dimer ([Cp * RhCl 2 ] 2 )0.01mmol, the oxidant bistrifluoromethanesulfonic acid imide silver salt (AgNTf 2 ) 0.06mmol, additive rhodium acetate (Rh(OAC) 2 ) 0.2mmol, 1,2 dichloroethane (DCE) 2ml, three times of argon gas exchange, and react at 150°C for 48h. After the reaction of the raw materials was detected by TLC, the reaction solution was cooled to room temperature, added with water and dichloromethane for extraction, and the organic phase was retained. The organic phase was dried with saturated saline and anhydrous sodium sulfate, concentrated under reduced pressure, and separated on a silica gel column to obtain the product I. That is, 5-methyl-2-(2 H -1,2,3-triazole) benzonitrile, the conversion rate is 92%, and the yield is 76%.
[0045]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com