A method for electrochemically synthesizing lactone
An electrochemical and lactone technology, applied in the field of electrochemical synthesis of lactone, can solve the problems of cumbersome process, unfavorable industrial operation and large-scale production, and achieve the effect of simple process, easy control and increased versatility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] (1) Add 40g of raw material 2-phenylbenzoic acid (1aa), 6.6g of electrolyte tetra-n-butylammonium tetrafluoroborate and 50mL of solvent acetonitrile into the electrolytic cell without diaphragm, and then insert the graphite electrode with a current density of 20mA / cm 2 The constant current at 20 o Stirring reaction under C;
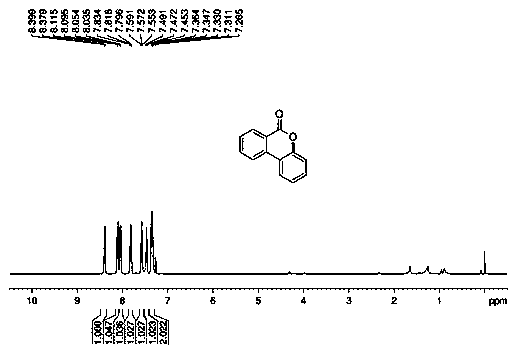
[0052] (2) The reaction was tracked by thin layer chromatography. After the reaction was completed, the solvent was spun off under vacuum, and then the lactone product 6 was obtained by recrystallization and separation. H - Benzo[c]chroman-6-one (2aa) 33g, 84% yield.
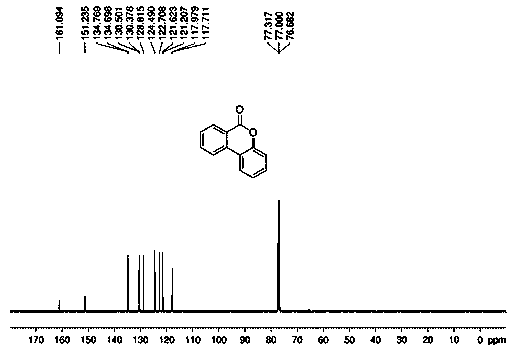
[0053] 1 H NMR (400 MHz, CDCl 3 ): δ 8.40-8.38 (d, J = 7.9 Hz, 1H), 8.11-8.09(d, J = 8.0 Hz, 1H), 8.05-8.03 (d, J = 7.8 Hz, 1H), 7.83-7.80 (t, J = 7.6 Hz,1H), 7.59-7.55 (t, J = 7.6 Hz, 1H), 7.49-7.45 (t, J = 7.7 Hz, 1H), 7.36-7.31(m, 2H); 13 C NMR (100 MHz, CDCl 3 ): δ 161.1, 151.2, 134.8, 134.7, 130.5, 130.4, 128.8, 124.5, 122.7, 121.6, 121.2, 118.0, 117.7.
Embodiment 2
[0055] (1) Add 108mg of raw material 2-(4-fluorophenyl)benzoic acid (1ab), 342mg of electrolyte tetra-n-butylammonium perchlorate and 10mL of solvent acetone into the electrolytic cell without diaphragm, then insert the platinum plate electrode, and pass The input current density is 10mA / cm 2 The constant current at 5 o Stirring reaction under C;
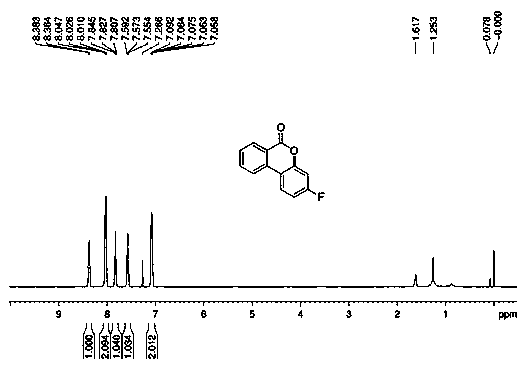
[0056] (2) Track the reaction by thin-layer chromatography. After the reaction is completed, the solvent is removed under vacuum, and then separated by silica gel column chromatography to obtain the lactone product 3-fluoro-6 H - Benzo[c]chroman-6-one (2ab) 67 mg, yield 63%.
[0057] 1 H NMR (400 MHz, CDCl 3 ): δ 8.38-8.36 (d, J = 7.8 Hz, 1H), 8.05-8.01(m, 2H), 7.85-7.81 (t, J = 7.6 Hz, 1H), 7.59-7.55 (t, J = 7.5 Hz, 1H), 7.09-7.06 (m, 2H); 13 C NMR (100 MHz, CDCl 3 ): δ 164.7-162.2 (d, J F-C = 249.6 Hz),160.7, 152.2-152.1 (d, J F-C = 12.5 Hz), 135.0, 134.2, 130.6, 128.7, 124.4-124.3 (d, J F-C = 9.8 Hz), 121.5, ...
Embodiment 3
[0059] (1) Add 123mg of raw material 2-(4-methylphenyl)-4-chlorobenzoic acid (1ar), 660mg of electrolyte tetraethylammonium tetrafluoroborate and 50mL of solvent ethanol into the electrolytic cell without diaphragm, and then insert Platinum sheet electrode with a current density of 13.3mA / cm 2 constant current at 50 o Stirring reaction under C;
[0060] (2) Track the reaction by thin-layer chromatography. After the reaction is completed, the solvent is removed under vacuum, and then separated by silica gel column chromatography to obtain the lactone product 3-methyl-9-chloro-6 H - Benzo[c]chroman-6-one (2ar) 55 mg, 45% yield.
[0061] 1 H NMR (400 MHz, CDCl 3 ): δ 8.34 (d, J = 1.7 Hz, 1H), 8.02-8.00 (d, J =8.6 Hz, 1H), 7.89-7.87 (d, J = 8.5 Hz, 1H), 7.75-7.73 (dd, J = 8.6 Hz, 1.9Hz, 1H), 7.17-7.15 (d, J = 7.0 Hz, 2H), 2.45 (s, 3H) ; 13 C NMR (100 MHz, CDCl 3 ): δ160.3, 151.1, 141.8, 135.0, 134.4, 133.5, 130.0, 125.9, 123.1, 122.5, 122.2, 118.0, 114.7, 21.4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com