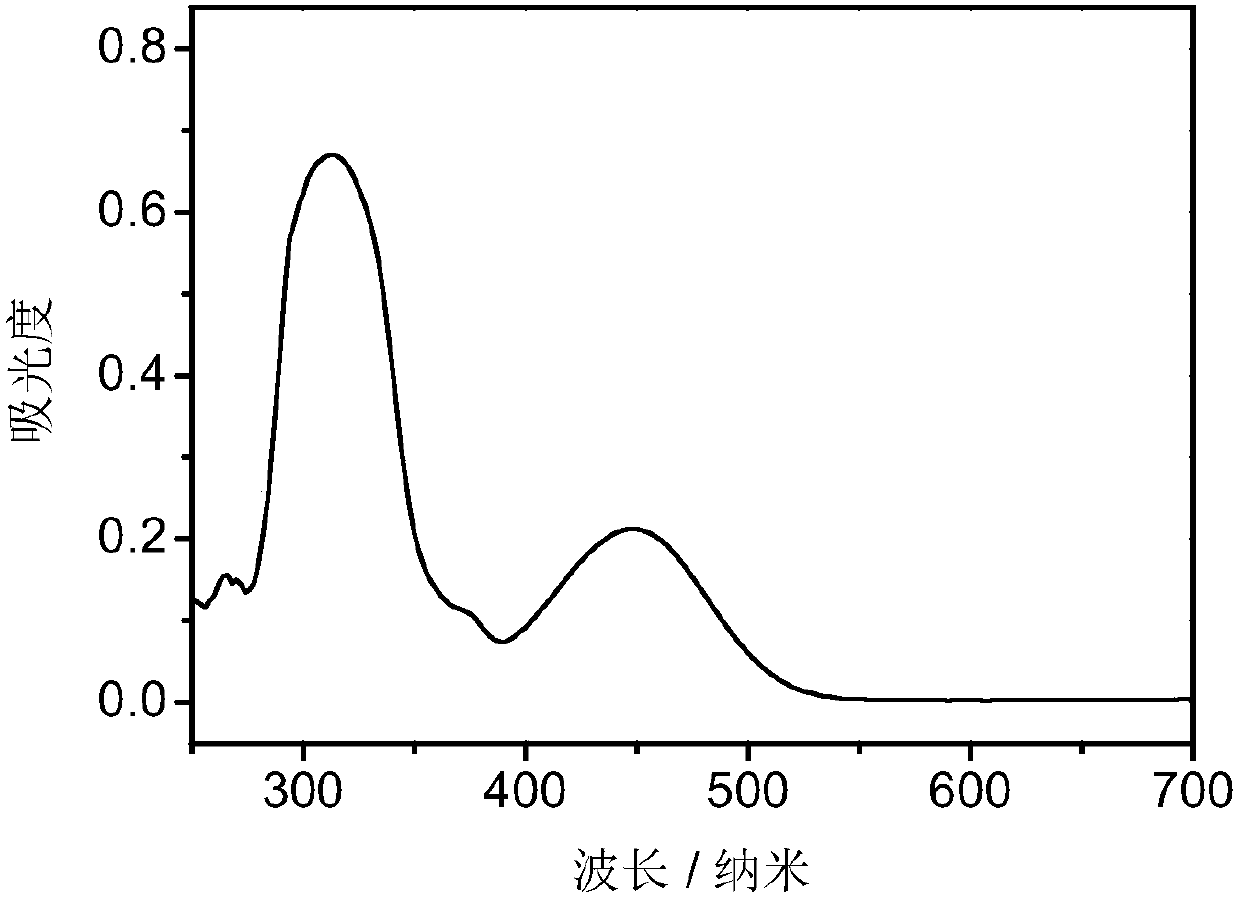
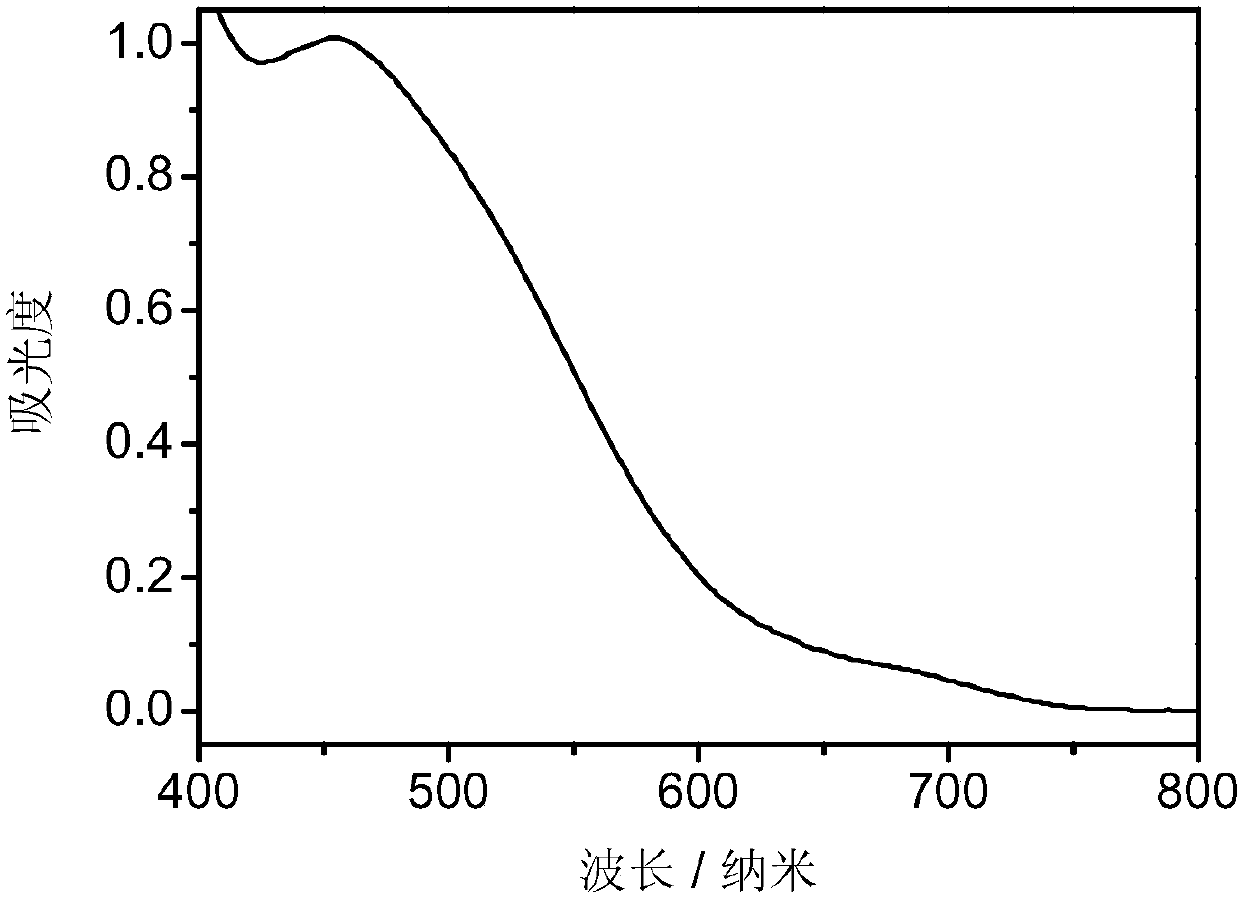
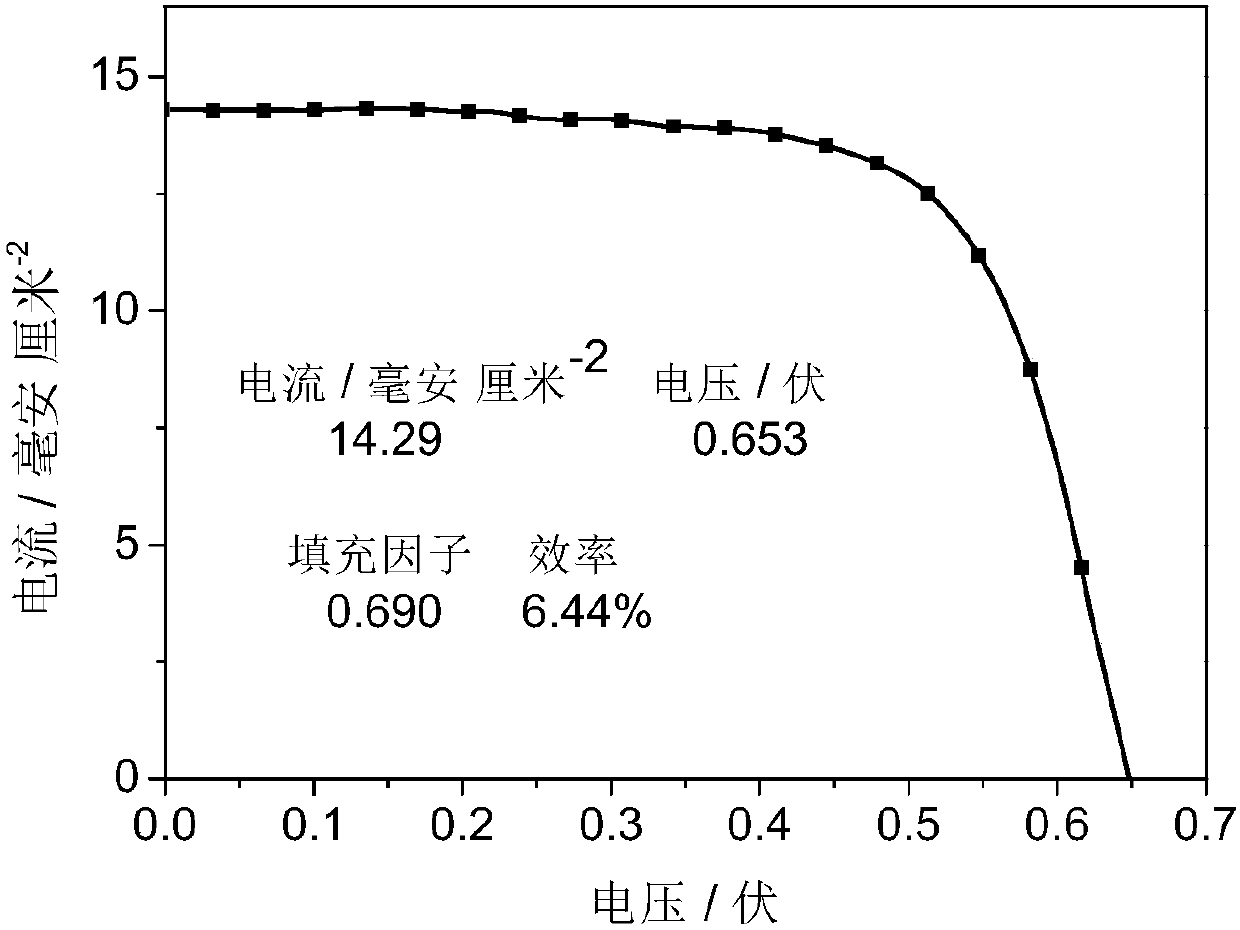
Carbazole compound and application thereof
A compound and carbazole technology, applied in the field of organic compounds, can solve the problems of narrow absorption spectrum, waste of solar energy, and limited application of carbazole organic molecules.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1 is synthesized (R 1 =R 2 =CH 3 , R 3 = benzene ring)
[0028]
[0029] In a 100mL one-mouth bottle, add 1.00g (5.99mmol) carbazole, dissolve it with tetrahydrofuran, place the one-mouth bottle in an ice bath and avoid light, then dissolve 2.67g (15mmol) NBS with tetrahydrofuran and pour it into constant pressure The dropping funnel was slowly dropped into a 100ml single-necked bottle, and stirred in an ice bath under the protection of nitrogen for 4 hours. The reactant was poured into 100mL water, extracted three times with ethyl acetate, the obtained liquid was spin-dried, and column chromatography was carried out with ethyl acetate:petroleum ether=1:200 to obtain 1.56g (4.8mmol) white solid, compound 2, yield 80%. 1 H NMR (500Hz, CDCl 3 ) 8.16 (s, 2H), 8.09 (s, 1H), 7.55 (d, J=8.5Hz, 2H), 7.33 (d, J=8.5Hz, 2H).
[0030]
[0031] In a 250mL single-necked bottle, add 3.35g (10.31mmol) of compound 2, 3.37g (24.76mmol) of compound 3, 5.66g (41.01...
Embodiment 2
[0044] Embodiment 2 is synthesized (R 1 =OC 8 h 17 , R 2 =CH 3 , R 3 = benzene ring)
[0045]
[0046] In a 250mL single-necked bottle, add 3.35g (10.31mmol) of compound 2, 6.19g (24.76mmol) of compound 13, 5.66g (41.01mmol) of sodium carbonate, 14ml of water, 26ml of ethanol, then add 30ml of solvent toluene, and then bubble for 30min Remove oxygen, then add 0.3g (0.26mmol) tetrakistriphenylphosphine palladium, reflux and stir at 80°C for 12h under the protection of nitrogen, add 20ml of dichloromethane after cooling, wash the organic phase with ice water 3 times, wash with anhydrous sodium sulfate After drying, the filtrate was filtered and evaporated to obtain a crude product, which was subjected to column chromatography with ethyl acetate:petroleum ether=1:200 to obtain 4.32 g (7.5 mmol) of compound 14 with a yield of 73%. 1 HNMR (500Hz, CDCl 3 )δ8.27(s,2H),8.06(s,1H),7.63(d,J=8.5Hz,6H),7.47(d,J=8.5Hz,2H),7.01(d,J=8.5Hz, 4H), 4.02(t, J=6.5Hz, 4H), 1.85-1.80(m, 4...
Embodiment 3
[0059] Embodiment 3 is synthesized (R 1 =OCH 3 , R 2 =CH 3 , R 3 = benzene ring)
[0060]
[0061] In a 250mL single-necked bottle, add 3.35g (10.31mmol) of compound 2, 3.8g (25mmol) of compound 22, 5.66g (41.01mmol) of sodium carbonate, 14ml of water, 26ml of ethanol, then add 30ml of solvent toluene, and then bubble for 30min to remove Oxygen, then add 0.3g (0.26mmol) tetrakistriphenylphosphine palladium, reflux and stir at 80°C for 12h under the protection of nitrogen, add 20ml of dichloromethane after cooling, wash the organic phase with ice water 3 times, and dry with anhydrous sodium sulfate , and then filtered and evaporated the filtrate to obtain a crude product, which was subjected to column chromatography with ethyl acetate:petroleum ether=1:200 to obtain 3.37g of compound 23 with a yield of 64%. 1 H NMR (500Hz, CDCl 3 )δ8.27(s,2H),8.06(s,1H),7.63(d,J=8.5Hz,6H),7.47(d,J=8.5Hz,2H),7.01(d,J=8.5Hz, 4H), 3.77(s, 6H).
[0062]
[0063] In a 100mL single-neck...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com