Method for carrying out bio-orthogonal polysaccharide labeling from microbiome level, and applications thereof
A bio-orthogonal and microbial technology, applied in the field of chemical biology, can solve the problems that bacteria cannot be studied, the operation is cumbersome, and they are helpless
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Three mice from clean-grade (specific-pathogen-free, SPF) C57 / BL6 adult mice (8-12 weeks) were divided into three groups, with one mouse in each group. After the large intestine and small intestine were taken through aseptic operation, they were quickly ground with a tissue grinder and filtered with a sieve (70mesh). The filtrate was diluted and evenly spread on a special intestinal microbiome medium (modifiedGifu anaerobe media ) (see Table 1 for the culture media corresponding to the three groups), 1 mM non-natural sugar probes have been added to the culture media of the experimental group in advance. Bacteria were collected after culturing in an anaerobic environment at 37°C for 5-7 days, and a fluorescent group was coupled to a cycloaddition reaction of azide and alkyne catalyzed by copper.
[0044] Table 1:
[0045]
[0046]
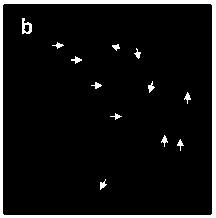
[0047] Afterwards, the samples were observed under a fluorescence microscope, and the observation results were as follows: Figure 1a...
Embodiment 2
[0052] Adopt the method identical with embodiment 1, use Ac 4 GalNAz or Ac 4 GlcNAz metabolically labeled bacterial populations (about 20mg), washed twice with PBS, lysed (sonicated or using bacterial lysate), centrifuged (10,000x g, 15min), supernatant was taken, and alkynyl biotin (0.1 mM), using copper to catalyze the cycloaddition reaction of azide and alkyne, after coupling the azide-labeled bacterial glycoprotein to biotin, the protein was precipitated with anhydrous methanol, centrifuged (4,000x g, 15min), and then used After washing with methanol for three times, use protein reconstitution solution to redissolve the protein, add strepavidin agarose microspheres to enrich the biotin-labeled protein, wash the microspheres thoroughly to remove non-specifically adsorbed protein, and finally use the loading buffer used for SDS-PAGE to load the protein The enriched glycoproteins are detached from the microspheres, and the proteins in the samples are analyzed by SDS-PAGE, an...
Embodiment 3
[0056] Use 8AzKDO to metabolically label the complete flora (20mg), in which Gram-negative bacteria are specifically labeled, and use PBS after coupling alkynyl-TAMRA (20μM) through the cycloaddition reaction of azide and alkyne catalyzed by copper. After washing 5 times, the finally obtained bacteria were resuspended in 500 μL PBS. Model mice (C57 / BL6) were intragastrically administered (200 μL) using this sample, and 4 to 6 hours later, the distribution of Gram-negative bacteria in the mouse intestine was observed. Observation methods include using two-photon microscopy to perform in vivo imaging of the flora in the intestinal tract of mice, or to isolate the intestinal tract of mice and perform frozen section processing, and use fluorescence microscopy to detect the presence of Gram-negative bacteria in the intestinal tract distribution is observed.
[0057] After the intestinal microbiome metabolically labeled with 8AzKDO probe (1mM) was administered to the mice, the smal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com