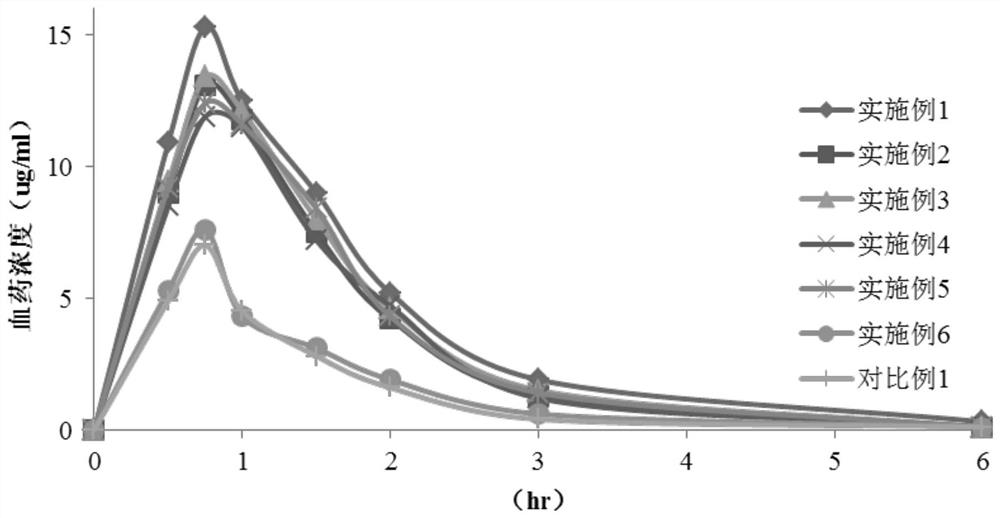
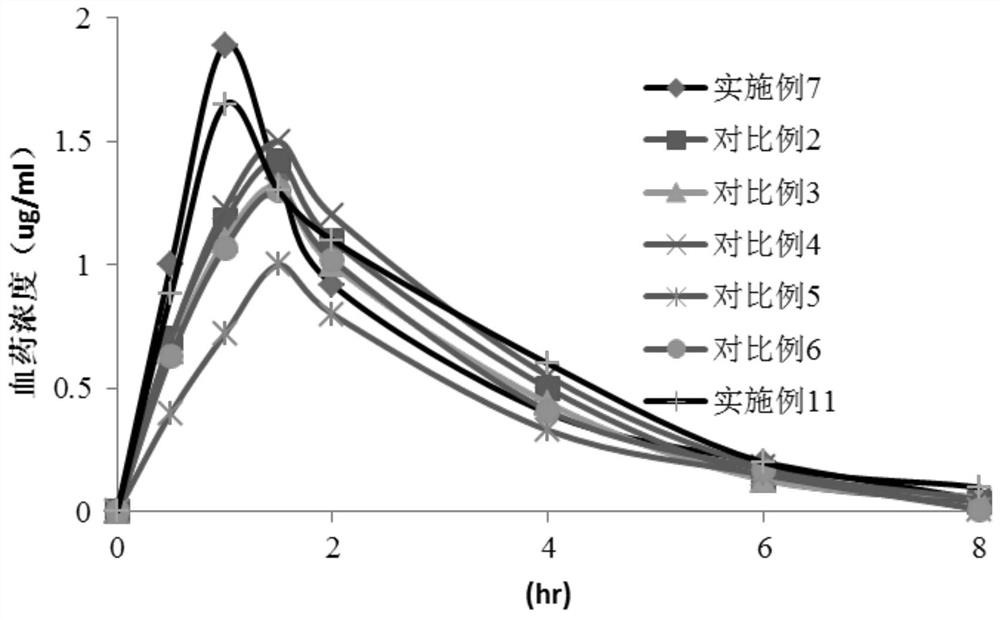
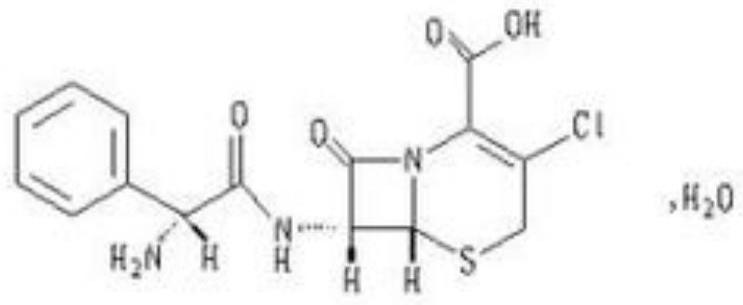
Cefaclor preparation and preparation method thereof
A technology for cefaclor and preparations, applied in the field of drug preparation, can solve problems such as poor absorption effect, and achieve the effects of high drug loading, sufficient and rapid disintegration, and promotion of dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0084] In the present invention, the preparation method comprising the cefaclor dispersible tablet preparation of sulbactam sodium liposome comprises the following steps:
[0085] Step 1), sulbactam sodium, egg yolk lecithin, cholesterol, dicetyl phosphate, and macromolecule polyethylene glycol are made into sulbactam sodium liposome;
[0086] Step 2), sieve the weighed cefaclor, emulsifier (such as polyethylene glycol 6000 and poloxamer 188), sulbactam sodium liposome and set auxiliary materials, mix, make soft material, pass Screen wet granules, dry, sieve and granulate;
[0087] Preferably, cefaclor and emulsifiers (such as polyethylene glycol 6000 and poloxamer 188) are pre-mixed, and then mixed with sulbactam sodium liposome and setting excipients.
[0088] Step 3), after adding lubricant and mixing, tableting to obtain cefaclor dispersible tablets.
[0089] Wherein, in step 2), the setting auxiliary materials include disintegrants, fillers, binders, swelling auxiliary ...
Embodiment 1
[0097] The preparation of embodiment 1 cefaclor dispersible tablet (do not contain sulbactam sodium)
[0098] The raw materials used are as follows:
[0099]
[0100] Adopt following production process to prepare cefaclor dispersible tablet:
[0101] (1), passing the weighed cefaclor, polyethylene glycol 6000, poloxamer 188 and the above-mentioned auxiliary materials through a 50-mesh sieve;
[0102] (2) After pre-mixing cefaclor with polyethylene glycol 6000 and poloxamer 188, mix it evenly with cross-linked polyvinylpyrrolidone, microcrystalline cellulose, hydroxypropyl methylcellulose and mannitol, Add water to make soft material, pass through 18-mesh sieve to granulate, dry at 60°C for 2 hours, and then granulate with 18-mesh sieve;
[0103] (3) Add magnesium stearate and pregelatinized starch, mix and compress to obtain cefaclor dispersible tablets, which contain 0.25g / tablet of cefaclor.
Embodiment 2
[0104] The preparation of embodiment 2 cefaclor dispersible tablets (do not contain sulbactam sodium)
[0105] The raw materials used are as follows:
[0106]
[0107] The production process of preparing cefaclor dispersible tablets is the same as that of Example 1, which contains 0.25 g / tablet of cefaclor, and the weight ratio of polyethylene glycol 6000 and poloxamer 188 is 1:1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com