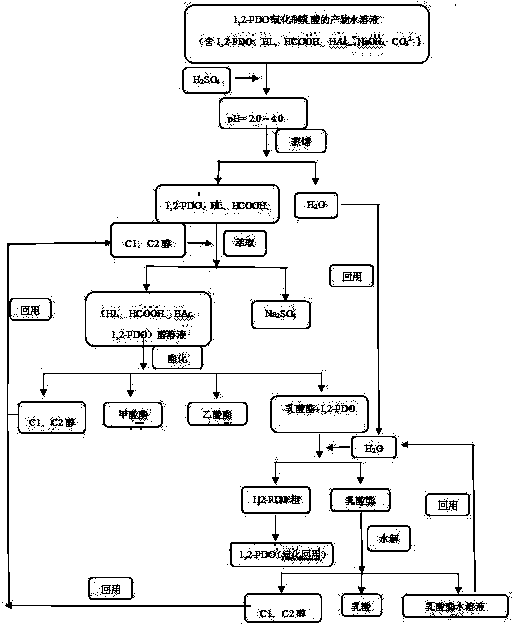
Product separation process for preparing lactic acid by means of oxidation of 1,2-propanediol
A technology for lactic acid and products, which is applied in the field of product separation technology for oxidizing 1,2-propylene glycol to lactic acid, can solve problems such as low efficiency and complicated process, and achieves reduction of separation energy consumption, cost saving, and increase of separation energy consumption and separation cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Single separation: 1,2-PDO oxidation product, containing (NaOH aqueous solution 60.0 mol %; unreacted 1,2-PDO 4.8 mol %; LA 25.0 mol %; HCOOH 1.2 mol %; HAc 6.2 mol %; CO 3 2- 2.8mol%). Add H first 2 SO 4 Acidify to pH = 2.0 to neutralize NaOH and CO 3 2- , vacuum rotary evaporation evaporates H 2 O (reuse as ester extraction agent); add CH 3 OH extraction (m(CH 3 OH): m (acid) = 0.5: 1.0 (g / g)), filtered, and the solid Na 2 SO 4 extracted; the alcohol filtrate was directly added to the reactor for esterification (70 °C, 12 h), during the esterification process methyl acetate, methyl formate and unreacted CH 3 OH can be distilled out directly, add pipetting agent H 2 O under vacuum and reduced pressure (0.5 kPa, 60 ℃) to remove methyl lactate (m(H 2 O):m(lactate)=2.0:1.0(g / g)), the remaining lactic acid continues to be esterified (70 ℃, 12 h), after esterification, add pipetting agent H 2 O(m(H 2 O):m(lactate)=2.0:1.0 (g / g)) removed; the residue was distil...
Embodiment 2
[0028] Single separation: 1,2-PDO oxidation product, containing (NaOH aqueous solution 80.0 mol %; unreacted 1,2-PDO 1.0 mol %; LA 13.0 mol %; HCOOH 1.0 mol %; HAc 3.0 mol %; CO 3 2- 2.0 mol%). H first 2 SO 4 Acidify to pH=2.0, neutralize NaOH and CO 3 2- , and the H 2 O (reuse as ester extraction agent); add C 2 h 5 OH extraction (m(C 2 h 5OH): m (acid) = 1.0 : 1.0 (g / g)), filter, the solid Na 2 SO 4 Extraction; the alcohol filtrate is directly added to the reactor for esterification (90°C, 10 h), during the esterification process ethyl acetate, ethyl formate and unreacted C 2 h 5 OH can be distilled out directly, add pipetting agent H 2 O under vacuum and reduced pressure (1.0 kPa, 65 ℃) to remove ethyl lactate (m(H 2 O):m(lactate)=5.0:1.0 (g / g)), the remaining lactic acid continues to be esterified (90 ℃, 10 h), after esterification, add pipetting agent H 2 O(m(H 2 O): m (lactate) = 5.0:1.0 (g / g)) removed; after removing ethyl lactate, the residue was disti...
Embodiment 3
[0030] Cyclic separation: 1,2-PDO oxidation product, containing (NaOH aqueous solution 61.0 mol %; unreacted 1,2-PDO10.0 mol%; LA15.5 mol%; HCOOH 5.0 mol%; HAc 3.5 mol%; CO 3 2- 5.0 mol%). H first 2 SO 4 Acidify to pH = 4.0 to neutralize NaOH and CO 3 2- , and the H 2 O (reuse as ester extraction agent); add CH 3 OH extraction (m(CH 3 OH): m (acid) = 3.0 : 1.0 (g / g)), filter, the solid Na 2 SO 4 extracted; the filtrate was directly added to the continuous reactor for esterification (70 °C, 12 h), methyl acetate, methyl formate and unreacted CH 3 OH can be distilled out directly, add pipetting agent H 2 O under vacuum and reduced pressure (10 kPa, 75 ℃) to remove methyl lactate (m(H 2 O): m (lactate) = 1.0 : 1.0 (g / g)), the remaining lactic acid continues to be esterified (70 ℃, 12 h), after esterification, add pipetting agent H 2 O(m(H 2 O): m (lactate) = 1.0 : 1.0 (g / g)) removed; the residue was distilled under reduced pressure (1.0 kPa, 100 ℃), and the unreacte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com