Preparation method for pentafluoroethyl trifluoromethyl ether
A technology of pentafluoroethyl trifluoromethyl ether and trifluoromethyl hypofluorite, which is applied in the field of preparation of fluorine-containing ether compounds, and can solve the problems of harsh reaction conditions and only 10-20% of perfluorinated organic compounds , to achieve the effect of increasing the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
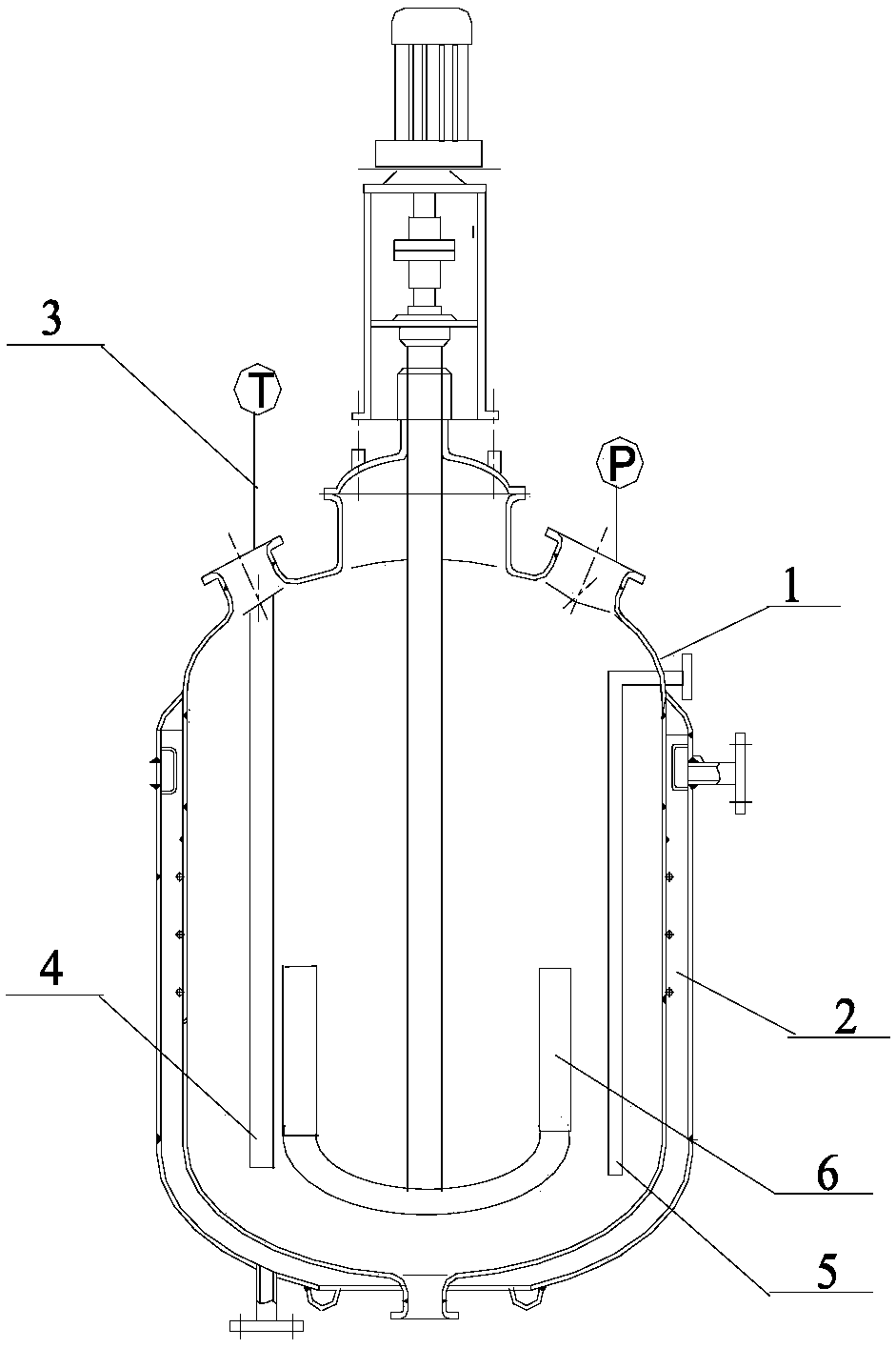
[0028] For the structure of the equipment for the reaction, see figure 1 , the material of the reaction kettle main body 1 is 304 stainless steel, and it is provided with a refrigerated medium jacket 2, a pressure monitoring point and a temperature monitoring device 3, and the temperature sensing part of the temperature monitoring device 3 extends below the liquid surface of the solvent; the tetrafluoroethylene feed pipe 4 and trifluoromethyl hypofluorite feed pipe 5 all lead to the bottom of the reactor, and the two feed pipes are on different sides. An anchor stirrer 6 is arranged in the reactor.
[0029] Inject 1.2L of liquid perfluoro-n-pentane into the reactor (capacity is 2L) at room temperature, then start the low-temperature circulation pump to pass the frozen alcohol into the jacket of the reactor, gradually cool down the reactor, and start the reactor The stirrer, the rotating speed is adjusted to 200r / min, and then the flow rate of 10L / h is blown into the reaction ...
Embodiment 2
[0032] Reaction equipment is with embodiment 1.
[0033] Inject 1.2L of liquid perfluoro-n-pentane into the reaction kettle at room temperature, then start the low-temperature circulation pump to pass the frozen alcohol into the freezing medium jacket of the reaction kettle, gradually cool down the reaction kettle, and start the agitator of the reaction kettle , the rotating speed is adjusted to 200r / min, and then the flow rate of 10L / h is blown into the reactor to replace with refined nitrogen (purity 99.99%, moisture content less than 20ppm), and after the temperature in the reactor drops to -85°C and stabilizes, Nitrogen was stopped, and tetrafluoroethylene (CF 2 = CF 2), after 20 minutes, trifluoromethyl hypofluorite (CF 3 OF) to react, the reaction tail gas is condensed and refluxed by the condenser, washed with 20% KOH lye, and then emptied, the reaction temperature is maintained at -82 ± 5 ° C, and the reaction is carried out at normal pressure for 2 hours. Stop feedi...
Embodiment 3
[0036] The reaction conditions were as in Example 1, the reaction solvent was replaced with dichloromethane, and the amount added was also 1.2 L. Other conditions remained unchanged. After the reaction, the purity of 98% pentafluoroethyltrifluoromethyl was obtained. 298 g of ether, the yield of pentafluoroethyl trifluoromethyl ether was 64.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com