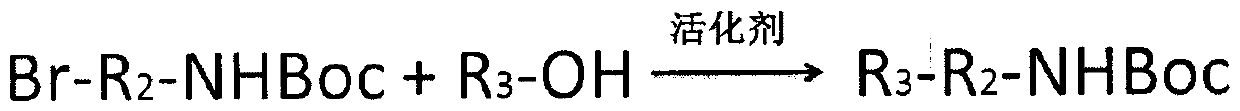Novel flavone-derived polymer nano-drug and application thereof to oncotherapy
A nano-drug and tumor treatment technology, applied in drug combinations, anti-tumor drugs, medical preparations of non-active ingredients, etc., can solve the problem of diffuse distribution, low selectivity, entry into tumor tissue, and unstable aggregation of small molecule photosensitizers, etc. problem, to achieve the effect of reducing systemic side effects, high drug loading, and prolonging circulation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1: Preparation of baicalein-desulfated heparin-derived polymer
[0072] Weigh 2-bromoethylamine hydrobromide and place it in an eggplant-shaped flask, add dichloromethane as a solvent, and then add di-tert-butyl dicarbonate. The molar ratio of di-tert-butyl dicarbonate to 2-bromoethylamine hydrobromide is 1.05:1. Separately weigh N,N-dimethyl-4-pyridinamine and pipette triethylamine into a 5mL EP tube, add 2mL of dichloromethane to dissolve, and slowly drop them into the above-mentioned eggplant-shaped bottle. The molar ratio of N,N-dimethyl-4-pyridinamine to 2-bromoethylamine hydrobromide is 2:5, and the molar ratio of triethylamine to 2-bromoethylamine hydrobromide is 2:1 . After reacting at room temperature for 40 minutes, transfer to a separatory funnel and wash with 0.1mol / L dilute sulfuric acid, saturated sodium bicarbonate solution and saturated sodium chloride solution three times respectively. After washing, the organic phase was transferred to a con...
Embodiment 2
[0076] Example 2: Preparation of chrysin-low molecular weight heparin derived polymer
[0077] Weigh 3-bromopropylamine hydrobromide and place it in an eggplant-shaped flask, add dichloromethane as a solvent, and then add di-tert-butyl dicarbonate. The molar ratio of di-tert-butyl dicarbonate to 3-bromopropylamine hydrobromide is 1.1:1. Separately weigh N,N-dimethyl-4-pyridinamine and pipette triethylamine into a 5mL EP tube, add 2mL of dichloromethane to dissolve, and slowly drop them into the above-mentioned eggplant-shaped bottle. The molar ratio of N,N-dimethyl-4-pyridinamine to 3-bromopropylamine hydrobromide is 1:5, and the molar ratio of triethylamine to 3-bromopropylamine hydrobromide is 1.05:1. After reacting at room temperature for 40 minutes, transfer to a separatory funnel and wash with 0.2 mol / L dilute hydrochloric acid, saturated sodium bicarbonate solution and saturated sodium chloride solution three times respectively. After washing, the organic phase was tra...
Embodiment 3
[0081] Example 3: Preparation of quercetin-unfractionated heparin-derived polymer
[0082] Weigh 4-bromo-1-butylamine hydrobromide and place it in an eggplant-shaped flask, add dichloromethane as a solvent, and then add benzyl chloroformate. The molar ratio of benzyl chloroformate to 4-bromo-1-butylamine hydrobromide is 3:1. Separately weigh 4-pyrrolidinylpyridine, absorb triethylamine into a 5mL EP tube, add 2mL dichloromethane to dissolve, and slowly drop into the above-mentioned eggplant-shaped bottle. The molar ratio of 4-pyrrolidinylpyridine to 4-bromo-1-butylamine hydrobromide is 3:5, and the molar ratio of triethylamine to 4-bromo-1-butylamine hydrobromide is 3:1. After reacting at room temperature for 1 h, it was transferred to a separatory funnel and washed three times with 0.1 mol / L dilute sulfuric acid, saturated sodium bicarbonate solution and saturated sodium chloride solution respectively. After washing, the organic phase was transferred to a conical flask, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com