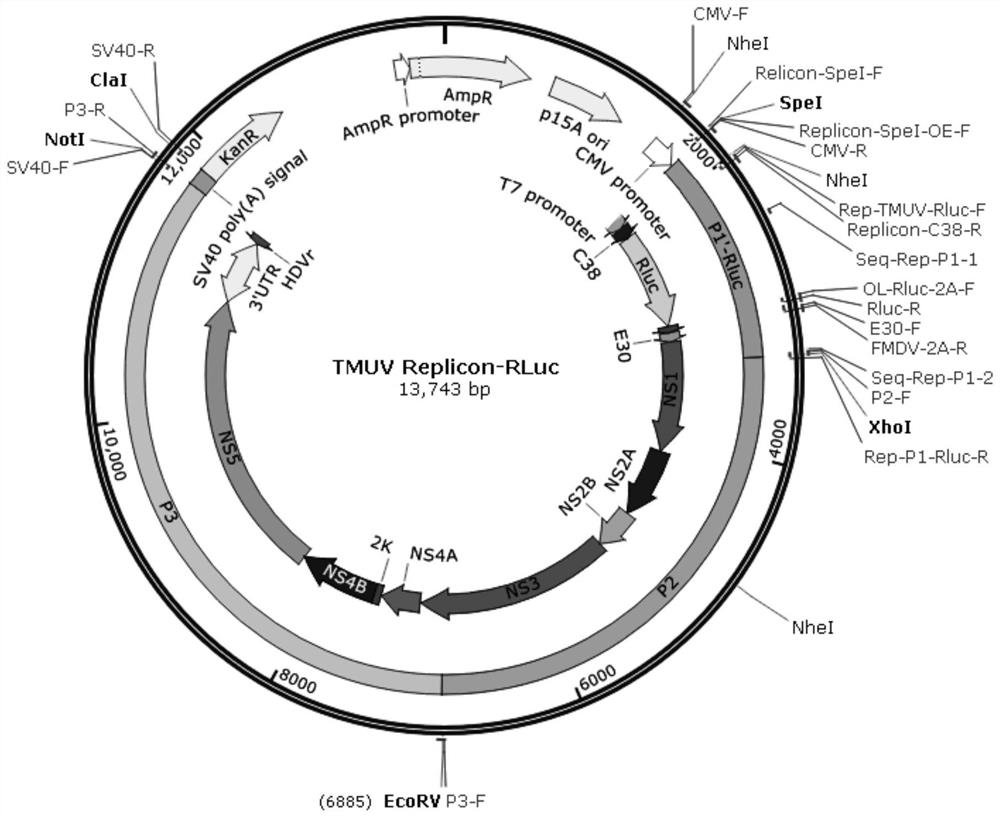
Replicon for transient transfection of duck Tembusu virus carrying Renilla luciferase and its construction method and application
A technology of renilla luciferase and duck tambusu virus, which is applied in the field of transient transfection replicon construction, can solve the problem that the tambusu virus replicon has not been successfully constructed, and achieves the effects of excellent sensitivity and convenient use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1, the construction of the Tembusu virus replicon vector carrying Renilla luciferase
[0033] 1. Construction of pACYC177B plasmid
[0034] Using pEGFP-C2 plasmid as template, SEQ ID No.3 (CMV-F: 5'-gccagtatacactccgctagcgtgatgcggttttggcagtac-3') and SEQ ID No.4 (CMV-R: 5'-ttccggctcgagcggactagtagctctgcttatatagacctccc-3') as primers, PCR amplification CMV was obtained by using SEQ ID No.5 (SV40-F: 5'-atatcaaggcaaaaagcggccgcaacttgtttattgcagcttataat-3') and SEQ ID No.6 (SV40-R: 5'-cgggcttcccatacaatcgattaagatacattgatgagtttggac-3') as primers, PCR amplification to obtain SV40, Amplified products were recovered and purified.
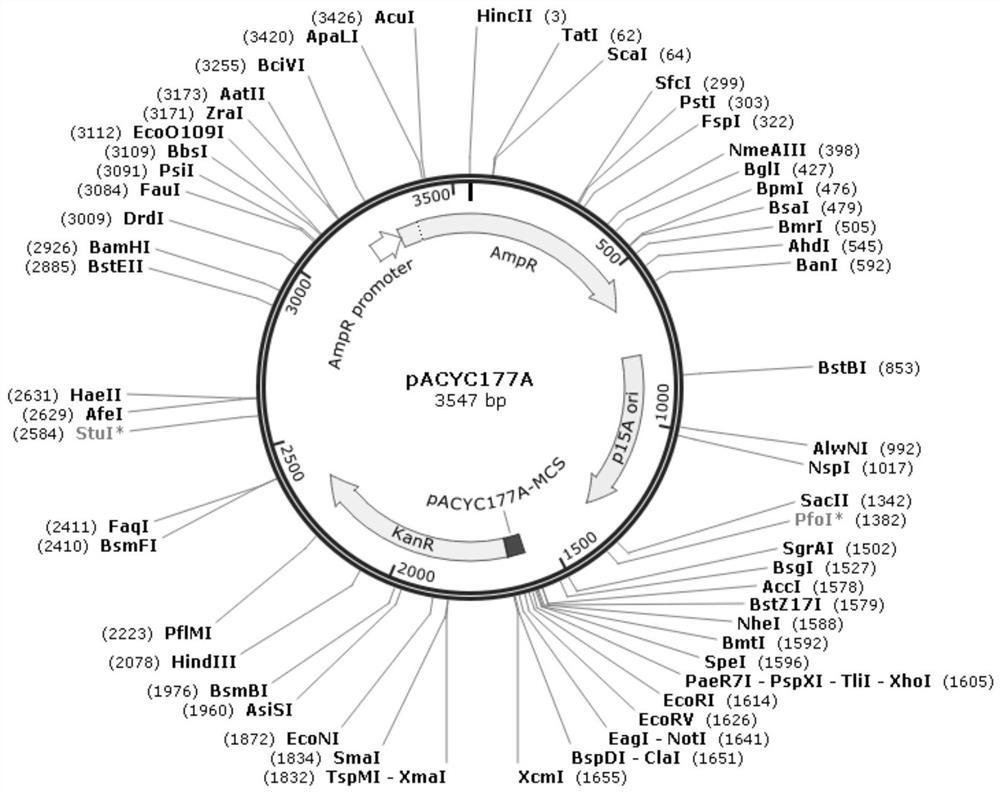
[0035] The pACYC 177A plasmid ( figure 1 ) for linearization by double enzyme digestion, the pACYC 177A plasmid sequence is shown in SEQ ID No.7. Using the ClonExpress II One Step Cloning Kit, the linearized pACYC 177A was ligated with the amplified CMV and transformed into SURE Escherichia coli. After the colonies grew out, the bacteria we...
Embodiment 2
[0052] Embodiment 2, RLuc reporter gene activity detection
[0053] A method for detecting RLuc reporter gene activity, comprising the steps of:
[0054] 1) Inoculate BHK21 cells in good growth state into 48-well cell culture plates;
[0055] 2) Grow for 12-24 hours, and when the cells grow to a confluence of 70-90%, transfect the pACYC-Rep-TMUV-Rluc plasmid according to the instructions of TransIntro ELTransfection Reagent.
[0056] 3) 4h, 8h, 12h, 24h, 36h, 48h, 72h, 96h, and 120h after transfection, measure the activity of the reporter gene Rluc according to the instructions of the TransDetect Single-Lucifersase (Renilla) Reporter Assay Kit. The specific operations are as follows:
[0057] a) Discard the cell culture medium, carefully rinse the cells twice with PBS, add 60 μl Cell Lysis solution, lyse at room temperature for 10 minutes, scrape the cells into an EP tube, centrifuge at 12000g at 4°C for 10 minutes, and take the supernatant for later use;
[0058] b) Add the...
Embodiment 3
[0059] Embodiment 3, RLuc activity detection
[0060] Using mMESSAGE mMACHINE TM T7Transcription Kit performs in vitro transcription, strictly following the supplier’s recommended procedures, using RNase-free pipette tips and EP tubes:
[0061] (1) In order to prevent the transcription of an excessively long chain, NotI single enzyme digestion fully linearized 10 μg of pACYC-Rep-TMUV-Rluc plasmid to terminate transcription;
[0062] (2) Preparation of in vitro transcription system:
[0063]
[0064] Then incubate at 37°C for 3h;
[0065] (3) After the transcription is completed, add 1 μl TuRBO DNase, mix well, and incubate at 37°C for 15 minutes;
[0066] (4) Lithium chloride precipitation to recover RNA:
[0067] a) Add 30μl Nuclease-free ddH 2 O and 30 μl LiCl precipitation solution, mix gently, and place at -20°C for at least 30 minutes;
[0068] b) Centrifuge at 4°C for 15 minutes, ≥12000 rpm / min;
[0069] c) Aspirate and remove the supernatant, add 1ml of 70% e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com