Preparation method of high-activity electrode having precious-metal-modified surface of bimetal nanocomposite material
A technology of bimetallic nanocomposite materials is applied in the field of preparation of highly active electrodes on the surface of precious metal modified bimetallic nanocomposite materials, and achieves the effects of high catalytic activity, high peak current density and improved catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
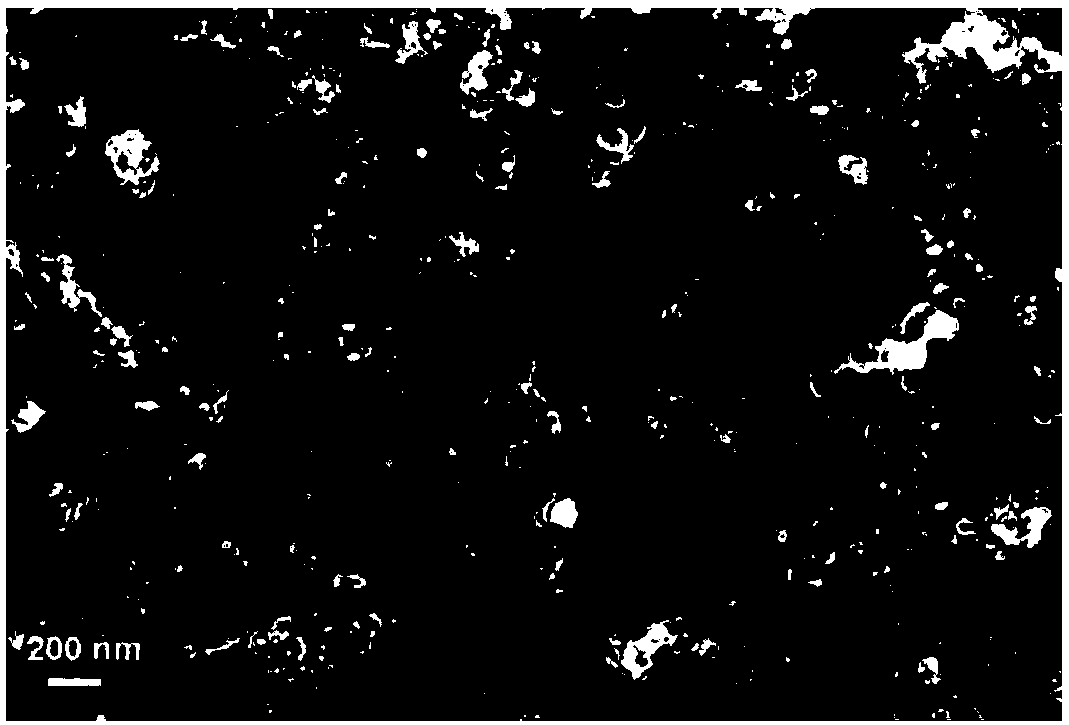
[0028] (1) Pt 1 Ni 3 / Carbon black electrode preparation. 1.2 mg of carbon black was taken into 19 mL of double distilled water, and ultrasonically treated for 20 minutes to obtain a black carbon black suspension. Then add 37.5μL of 0.1M Ni(NO 3 ) 2 solution and 12.5μL of 0.1MH 2 PtCl 6 solution, and continue to ultrasonically vibrate for 20 minutes to obtain a solution containing Ni(NO 3 ) 2 and H 2 PtCl 6 carbon black suspension. Add 1 mL of freshly prepared 0.1 M NaBH to the above liquid under magnetic stirring 4 solution and continued stirring for 40 minutes. After that, the sample was transferred to a test tube and left to settle for 12 hours. After layering, the supernatant was removed, and 10 μL of Nafion solution with a mass fraction of 5% was added, and ultrasonically treated for 5 minutes to obtain 0.5 mL of black ink-like Pt 1 Ni 3 / carbon black suspension. 0.3μm and 0.05μm Al were used successively 2 O 3 Glassy carbon electrodes with a diameter of...
Embodiment 2
[0032] On the basis of Example 1, Au was prepared 0.06 / Pt 1 Ni 3 / Carbon black electrodes.
[0033] (1) Pt 1 Ni 3 / Carbon black electrode preparation. It is the same as the procedure of (1) in Example 1.
[0034] (2)Au 0.06 / Pt 1 Ni 3 / Carbon black electrode preparation. The same procedure as (2) in Example 1, but the Au deposition time was 5 seconds. According to the amount of charge in the deposition process, the Au deposition amount is calculated to be about 1.19 μg cm -2 , to obtain Au 0.06 / Pt 1 Ni 3 / Carbon black electrodes.
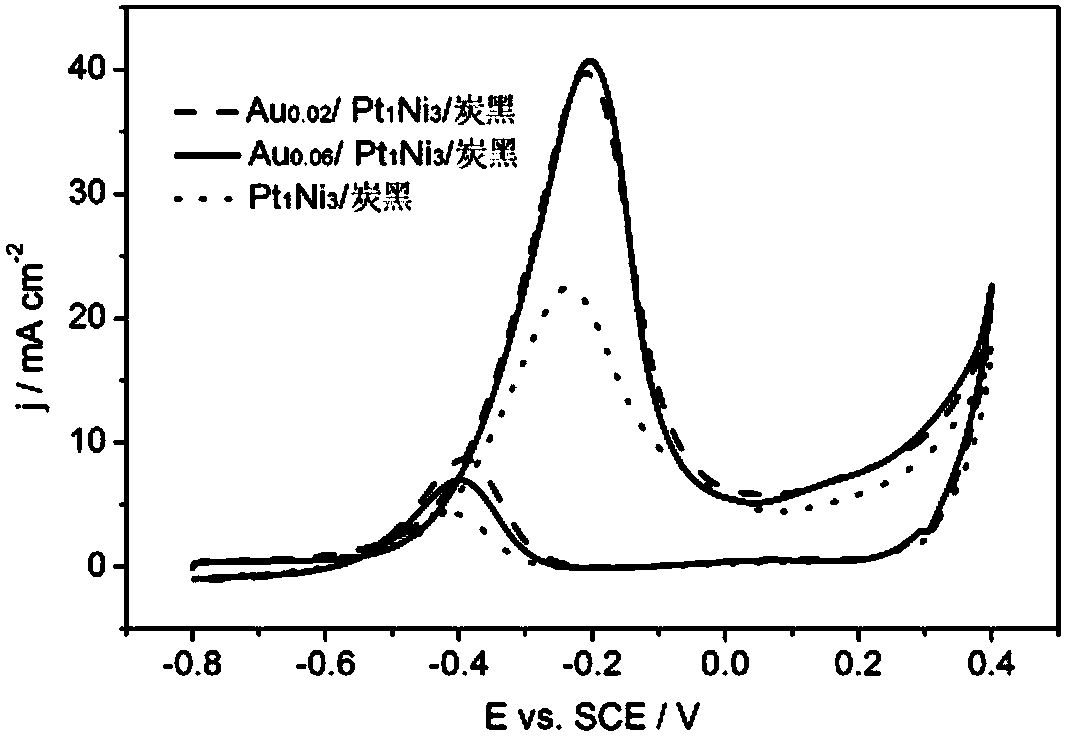
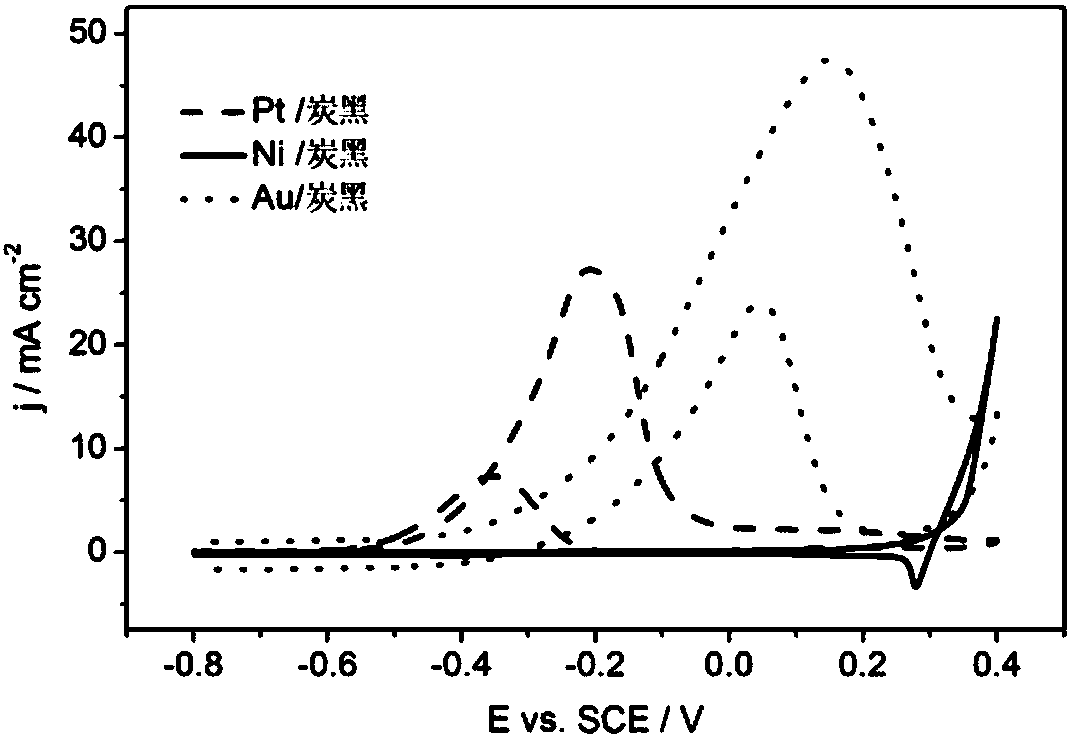
[0035] (3)Au 0.06 / Pt 1 Ni 3 Catalytic activity of carbon black electrodes for ethylene glycol oxidation in alkaline media. The same steps as (3) in Example 1, but the working electrode is Au 0.06 / Pt 1 Ni 3 / carbon black electrode, cyclic voltammetry was performed, and the results were as follows figure 2 shown. Au 0.06 / Pt 1 Ni 3 The peak potential of ethylene glycol oxidation reaction on carbon black electrode is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com