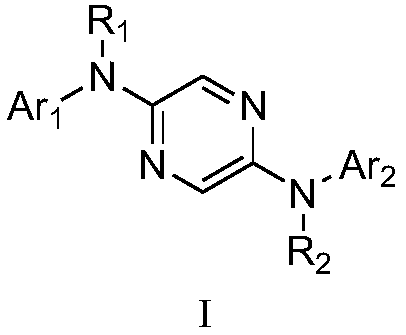
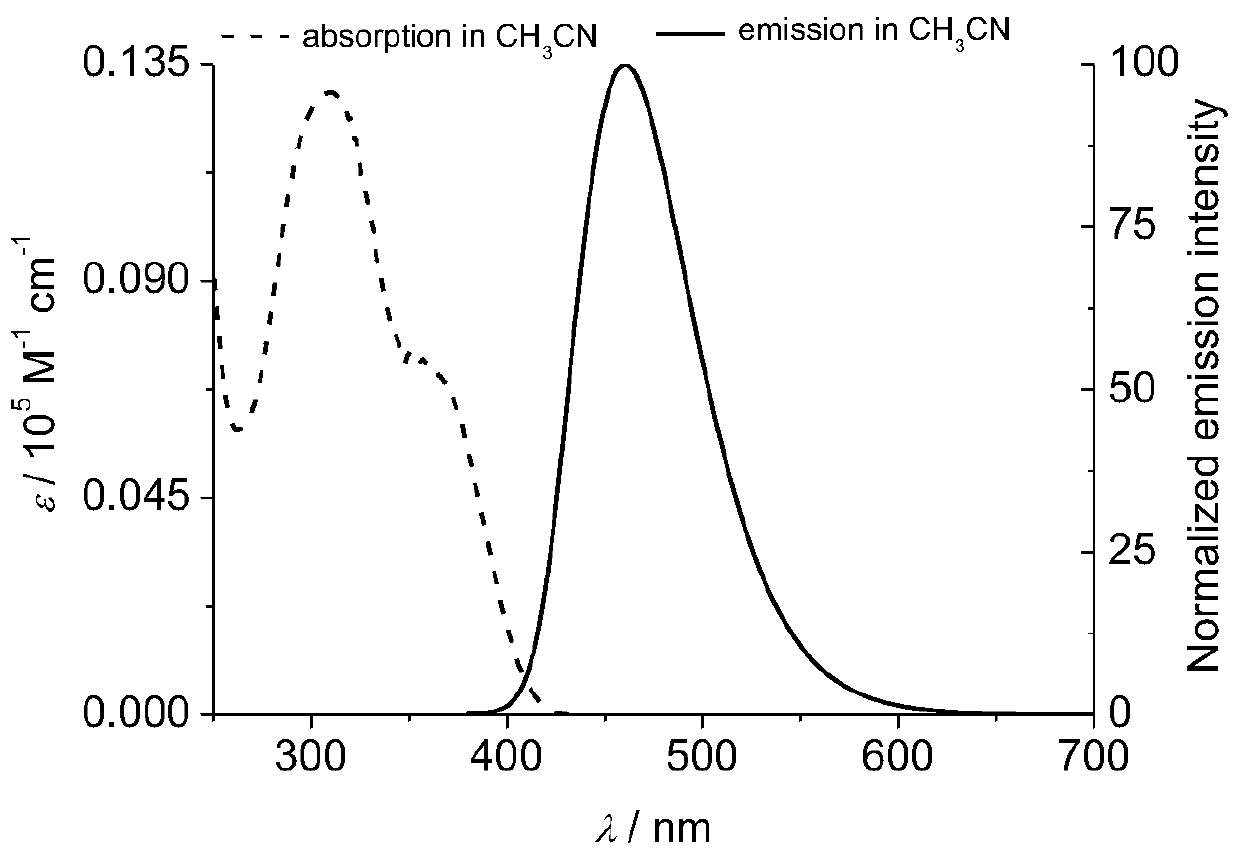
A kind of organic blue light-emitting material and preparation method thereof
A blue light-emitting material and organic technology, applied in the direction of light-emitting materials, organic chemistry, chemical instruments and methods, etc., can solve the problems that limit the development of organic light-emitting diode devices, achieve improved hole transport ability, simple structure, and high luminous efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment one: the synthesis of compound 1:
[0036]
[0037] Under nitrogen atmosphere, 476mg (2.0mmol) 2,5-dibromopyrazine, 183mg (0.2mmol) tris(dibenzylideneacetone) dipalladium, 110mg (0.2mmol) 1,1'-bis-diphenyl 768mg (8.0mmol) of phosphinoferrocene and sodium tert-butoxide were added to a pressure-resistant reaction flask. After the gas was pumped twice, 475mg (4.4mmol) of 2-(methylamino)pyridine was also added to the reaction flask under the protection of nitrogen. In the bottle, add toluene (15mL) again and start heating and reflux reaction, the temperature is controlled at 120 ° C, stop the reaction after 20h of reaction, cool to room temperature, remove the insoluble matter in the mixed solution after the reaction by suction filtration, remove the solvent in the obtained filtrate, and The obtained crude product was passed through a silica gel column to obtain 576 mg of compound 1 as a yellow solid, yield: 98%.
Embodiment 2
[0038] Embodiment two: the synthesis of compound 1:
[0039] Under nitrogen atmosphere, 119 mg (0.5 mmol) 2,5-dibromopyrazine, 46 mg (0.05 mmol) tris(dibenzylideneacetone) dipalladium, 28 mg (0.05 mmol) 1,1'-bis-diphenyl Add 192mg (2mmol) of phosphinoferrocene and sodium tert-butoxide into a pressure-resistant reaction flask, and after pumping out the gas twice, add 119mg (1.1mmol) of 2-(methylamino)pyridine to the reaction flask under nitrogen protection Add toluene (10mL) again and start heating to reflux reaction, the temperature is controlled at 100 ° C, stop the reaction after 24 hours of reaction, cool to room temperature, remove the insoluble matter in the mixed solution after the reaction by suction filtration, remove the solvent in the obtained filtrate, and The obtained crude product was passed through a silica gel column to obtain 135 mg of compound 1 as a yellow solid, yield: 93%.
Embodiment 3
[0040] Embodiment three: the synthesis of compound 1:
[0041] Under nitrogen atmosphere, 476mg (2.0mmol) 2,5-dibromopyrazine, 183mg (0.2mmol) tris(dibenzylideneacetone) dipalladium, 110mg (0.2mmol) 1,1'-bis-diphenyl 768mg (8.0mmol) of phosphinoferrocene and sodium tert-butoxide were added to a pressure-resistant reaction flask. After the gas was pumped twice, 475mg (4.4mmol) of 2-(methylamino)pyridine was also added to the reaction flask under the protection of nitrogen. In the bottle, add toluene (20mL) again and start the heating and reflux reaction, the temperature is controlled at 150 ° C, stop the reaction after 48 hours of reaction, cool to room temperature, remove the insoluble matter in the mixed solution after the reaction by suction filtration, remove the solvent in the obtained filtrate, and The obtained crude product was passed through a silica gel column to obtain 560 mg of compound 1 as a yellow solid, yield: 96%.
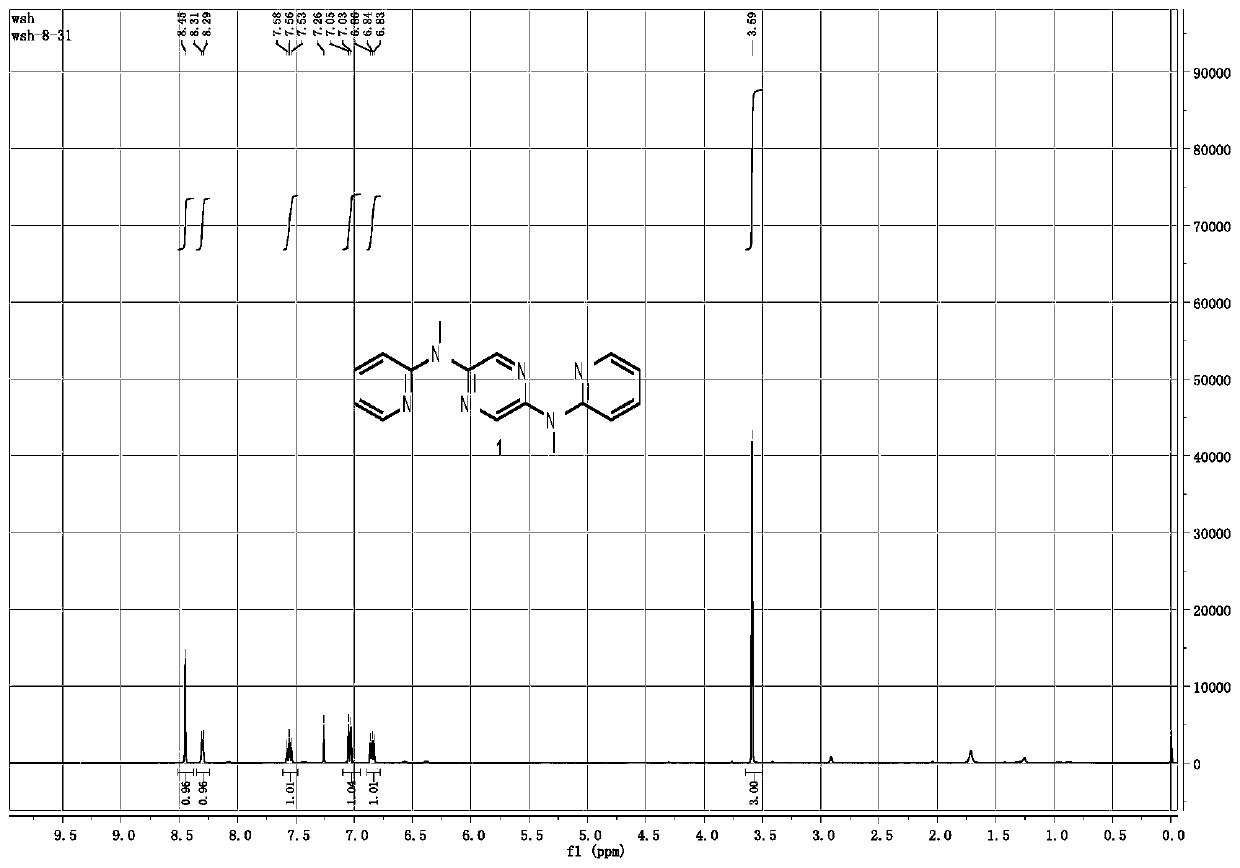
[0042] 2. Structural analysis
[0043] The H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com