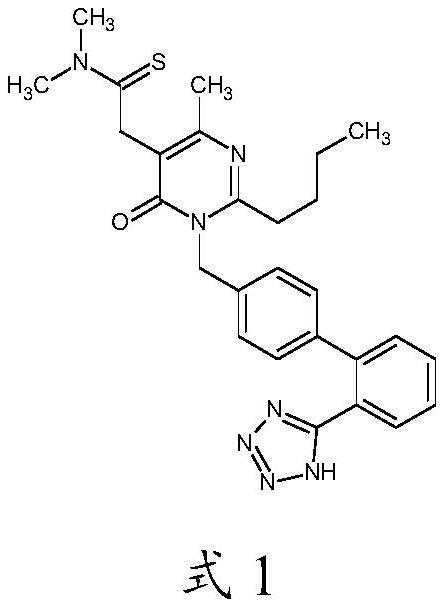
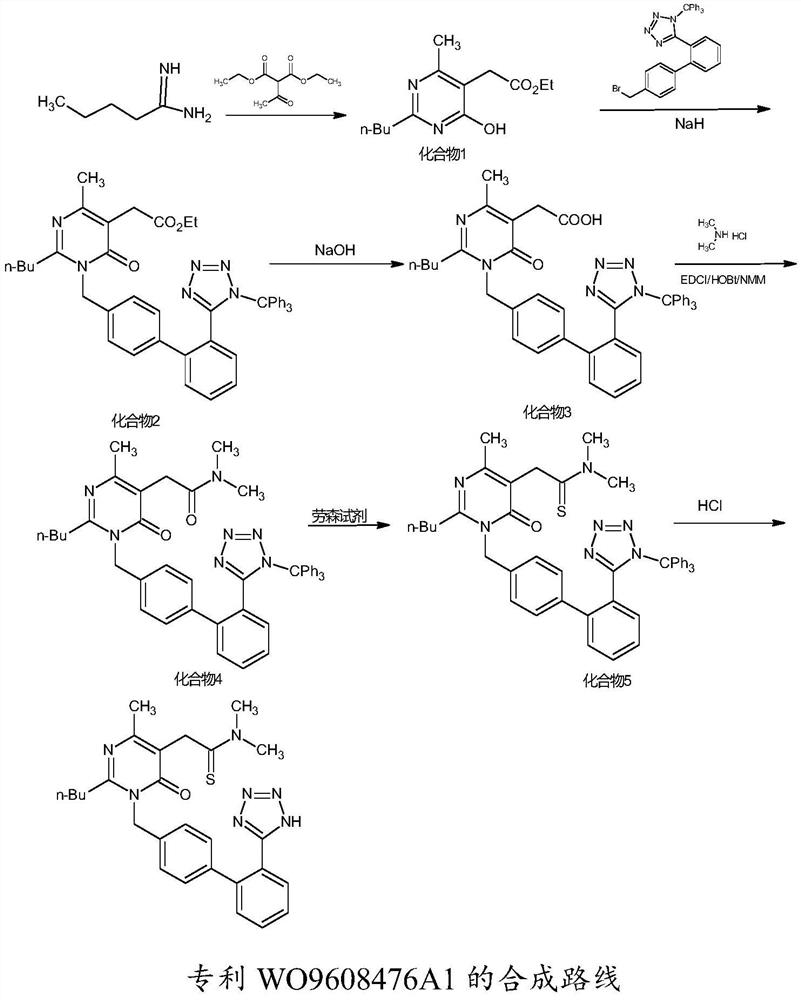
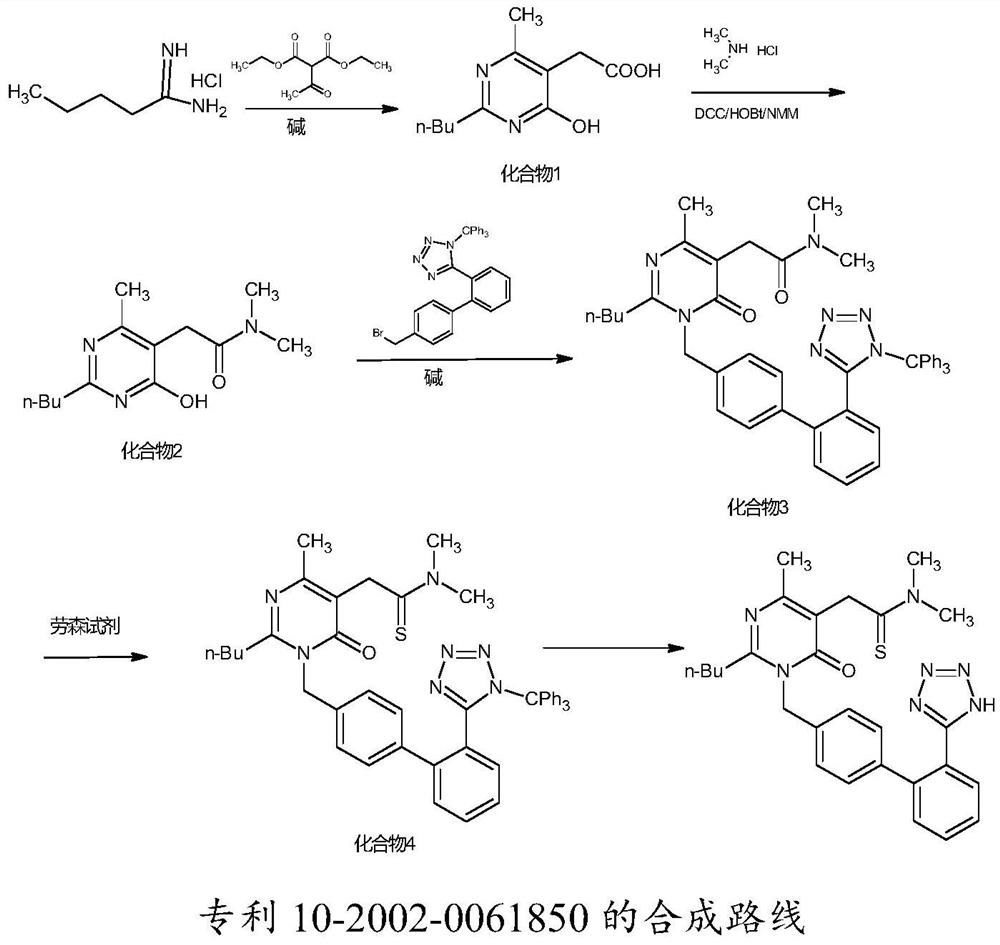
A kind of synthetic method of Fimasartan
A synthetic method, fimasartan technology, applied in the field of pharmaceutical synthesis, can solve the problems of unfavorable industrial production, difficulty in complete reaction of raw materials, and low atom economy, so as to avoid the use of silica gel and solvents, reduce process costs, and facilitate industrialization The effect of production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0054] Preparation example 1, prepare 2-(N,N-dimethylaminocarbonylmethyl) methyl acetoacetate (V) with sodium amide as base
[0055]
[0056] Under the protection of nitrogen, 81.92 g (2.10 mol) of sodium amide was added to 2000 ml of toluene, the stirring was started, and 232.22 g (2.00 mol) of methyl acetoacetate was added dropwise within 1 hour. After completion, the reaction was stirred at 80°C for 1 hours, a white suspension was obtained. Heating was stopped, and 243.14 g (2.00 mol) of 2-chloro-N,N-dimethylacetamide was added dropwise within 1 hour, and then refluxed for 3 hours. The reaction solution was concentrated to remove toluene, then 1300 ml of dichloromethane and 1300 ml of purified water were added thereto, stirred and the organic layer was separated. The organic layer was concentrated and purified by chromatography with a mixed solvent of ethyl acetate and n-hexane (v / v=1:5) to obtain 370 g of light yellow transparent oil (92% yield, 99.5% purity).
[0057...
preparation example 2
[0058] Preparation example 2, using lithium hydride as base to prepare 2-(N,N-dimethylaminocarbonylmethyl) methyl acetoacetate (V)
[0059]
[0060] Under the protection of nitrogen, 16.70 g (2.10 mol) of lithium hydride was added to 2000 ml of toluene, the stirring was started, and 232.22 g (2.00 mol) of methyl acetoacetate was added dropwise within 1 hour. After completion, the reaction was stirred at 80°C for 1 hours, a white suspension was obtained. Heating was stopped, and 243.14 g (2.00 mol) of 2-chloro-N,N-dimethylacetamide was added dropwise within 1 hour, and then refluxed for 3 hours. The reaction solution was concentrated to remove toluene, then 1300 ml of chloroform and 1300 ml of purified water were added thereto, stirred and the organic layer was separated. The organic layer was concentrated and purified by chromatography with a mixed solvent of ethyl acetate and n-hexane (v / v=1:5) to obtain 366.22 g of light yellow transparent oil (91% yield, 99.3% purity). ...
preparation example 3
[0062] Preparation Example 3, Preparation of 2-(N,N-dimethylaminocarbonylmethyl) ethyl acetoacetate (V)
[0063]
[0064] Under the protection of nitrogen, 81.92 grams (2.10 mol) of sodium amide was added to 2000 ml of toluene, and then under stirring, 260.28 grams (2.00 mol) of methyl acetoacetate was added dropwise within 1 hour. After completion, the reaction was stirred at 80°C After 1 hour, a white suspension was obtained. Heating was stopped, and 243.14 g (2.00 mol) of 2-chloro-N,N-dimethylacetamide was added dropwise within 1 hour, and then refluxed for 3 hours. The reaction solution was concentrated to remove toluene, then 1300 ml of chloroform and 1300 ml of purified water were added thereto, stirred and the organic layer was separated. The organic layer was concentrated and purified by chromatography with a mixed solvent of ethyl acetate and n-hexane (v / v=1:5) to obtain 344.38 g of a colorless transparent oil (yield 80%, purity 99.0%).
[0065] 1 H-NMR (600MHz,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com