Kit for simultaneously detecting 45 mutation sites of human EGFR (Epidermal Growth Factor Receptor) gene
A technology for detecting human mutation sites, applied in the fields of biotechnology and medicine, can solve the problem of lack of relevant reports on mutation site detection, and achieve the effect of convenient standardized operation, fewer components, and standardized operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Taking 10 samples as an example to illustrate the use of this kit:
[0084] (1) Take out 12 8-tube tubes from the kit, place them on a 96-well plate, melt the detection reagent I and the positive quality control product PC, centrifuge briefly, and place them on ice;
[0085] (2) Take 12 single PCR tubes, mark 1-10 and NC, PC respectively, add 3 μl Taq DNA polymerase to each tube, add to the bottom of the tube, and place on ice; Add 27 μl of 10 DNA samples to be tested, negative quality control product NC and positive quality control product PC, pipette to mix, close the cap tightly and centrifuge briefly (Note: Do not use a vortex shaker when mixing; follow-up);
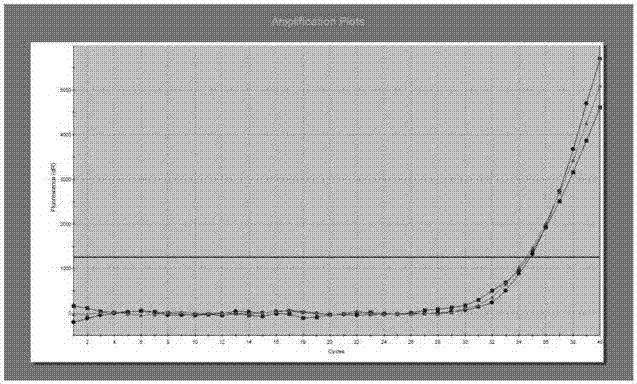
[0086] The positive quality control product PC is the TE solution (10mM Tris-HCl, 1mM EDTA, pH8.0) of the internal reference gene IR and human EGFR mutant gene plasmids (Table 1, * mark), wherein the final concentration of each plasmid is 5 ×10 -4 ng / μL.
[0087] Negative quality control product NC is a bu...
Embodiment 2
[0090] The detection limit experiment of EGFR gene mutation in the human EGFR wild-type genomic DNA of embodiment 2
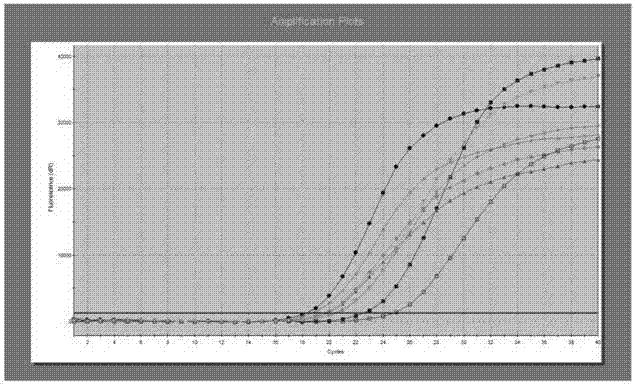
[0091] Get human EGFR wild-type genomic DNA, human EGFR mutant gene plasmids (Table 1, * mark) and dilution buffer, prepare human EGFR wild-type genomic DNA containing 20ng / μl at the same time, and the content is respectively wild-type 10%, 5% %, 1%, 0.5%, 0.4%, 0.3%, 0.2%, 0.1% of the human EGFR mutant gene plasmids of the detection limit reference; preparations containing 10ng / μl of human EGFR wild-type genomic DNA and the content were respectively Wild-type 10%, 5%, 1%, 0.5%, 0.4%, 0.3%, 0.2%, 0.1% detection limit reference substance of human EGFR mutant gene plasmids; preparation of human EGFR wild type containing 5ng / μl at the same time Genomic DNA and its contents are wild-type 10%, 5%, 1%, 0.9%, 0.8%, 0.7%, 0.6%, and 0.5% of the detection limit reference products of human EGFR mutant gene plasmids, and the positive detection rate is recorded (The result...
Embodiment 3
[0093] Example 3 Negative reference product coincidence rate experiment
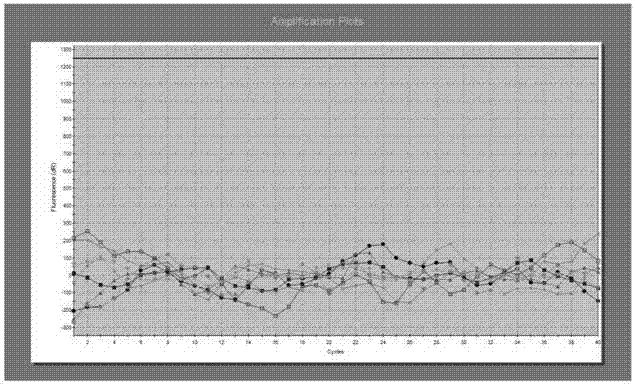
[0094] 10 cases of negative reference samples of 15ng / μl human EGFR wild-type genomic DNA were detected once each, and the negative detection status was recorded. It was found that the test results of all EGFR wild-type nucleic acid samples were negative, and the negative coincidence rate was 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com