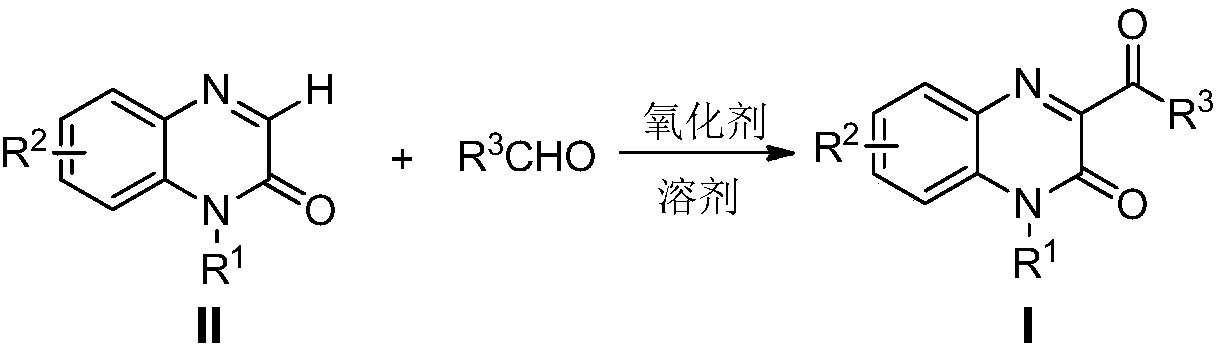
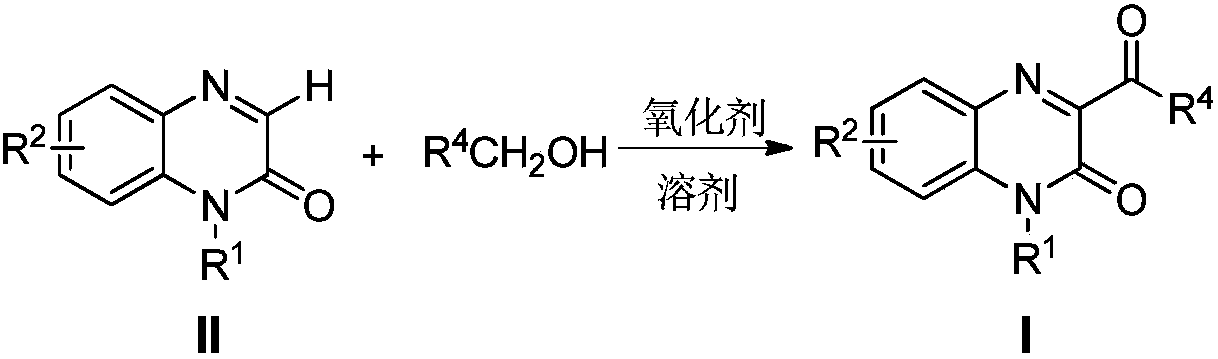
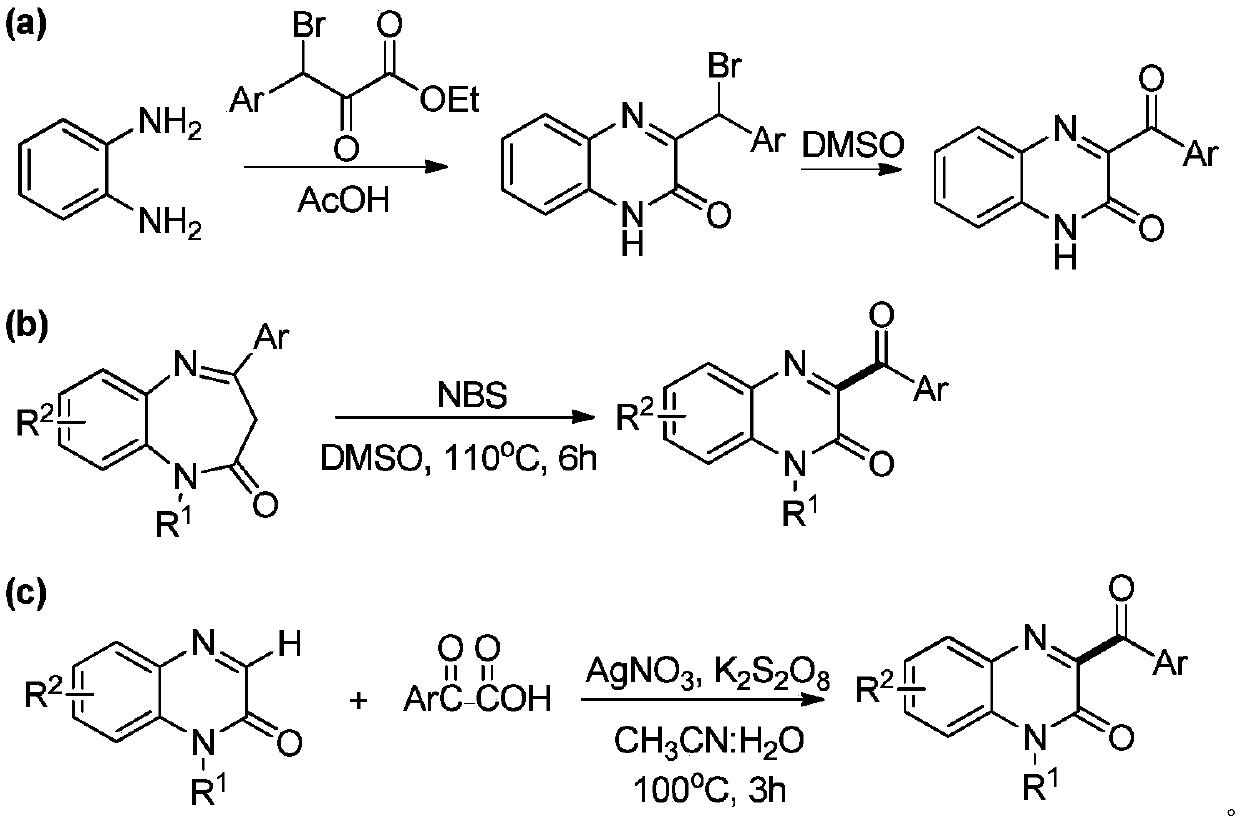
Method for preparing 3-acyl quinoxaline ketone derivative
A technology for acylquinoxalinone and derivatives, which is applied in the field of synthesis of 3-acylquinoxalinone derivatives, which can solve the problems that do not conform to the development of green chemistry, require transition metal catalysis, and difficult to obtain raw materials, etc., so as to avoid purification The effect of mild treatment and reaction conditions and a wide range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1.R 1 =-R 2 =-H, R 3 When =-Ph, the preparation of 3-(benzoyl)quinoxalin-2-one derivatives
[0020] Add quinoxalin-2-one (0.2mmol, 29.2mg) and benzaldehyde (0.4mmol, 42.4mg) in 25mL round-bottomed flask, then add mass percentage content 70% peroxy tert-butanol aqueous solution (0.8mmol, 104mg), and finally add 2mL of 1,2-dichloroethane as solvent. React at 70°C for 5 hours; after the reaction, remove the solvent under reduced pressure, add 10 mL of ethyl acetate to the residue, wash twice with 20 mL of saturated brine; wash the organic layer with anhydrous Na 2 SO 4 After drying and concentrating under reduced pressure, it was separated and purified by column chromatography (eluent: ethyl acetate / petroleum ether=1 / 2) to obtain 0.043 g of a colorless solid with a yield of 85.0%.
[0021]
[0022] Colorless solid, melting point mp 251-252°C. 1 H NMR(400MHz,DMSO)δ:12.88(s,1H), 7.98(d,J H-H =7.0Hz,2H),7.83(dd,J H-H =8.1Hz,J H-H =1.1Hz,1H),7.74(t,J H-H =...
Embodiment 2
[0023] Example 2.R 1 =-PhCH 2 , R 2 =-H,R 3 Preparation of 1-benzyl-3-(benzoyl)quinoxalin-2-one derivatives when =-Ph
[0024]Add 1-benzylquinoxalin-2-one (0.2mmol, 47.2mg) and benzaldehyde (0.4mmol, 42.4mg) in a 25mL round-bottomed flask, and then add 70% mass percent peroxy tert-butanol aqueous solution (0.8mmol, 104 mg), and finally 2mL of 1,2-dichloroethane was added as a solvent. React at 65°C for 6 hours; after the reaction, remove the solvent under reduced pressure, add 10 mL of ethyl acetate to the residue, wash twice with 20 mL of saturated brine; wash the organic layer with anhydrous Na 2 SO 4 After drying and concentrating under reduced pressure, it was separated and purified by column chromatography (eluent: ethyl acetate / petroleum ether=1 / 6) to obtain 0.054 g of a colorless solid with a yield of 80.0%.
[0025]
[0026] Yellow solid, melting point 128-129°C. 1 H NMR (400MHz, CDCl 3 )δ:8.01(dd,J H-H =8.0Hz,J H-H =1.4Hz,2H),7.92(dd,J H-H =8.4Hz,J H-H ...
Embodiment 3
[0027] Example 3.R 1 =-C 6 h 13 , R 2 =-H,R 3 When =-Ph, the preparation of 1-n-hexyl-3-(benzoyl)quinoxalin-2-one derivatives
[0028] Add 1-n-hexylquinoxalin-2-one (0.2mmol, 46.0mg) and benzaldehyde (0.4mmol, 42.4mg) in a 25mL round bottom flask, then add di-tert-butoxy peroxide (0.6mmol, 87.6 mg), and finally add 2 mL of acetone as a solvent. React at 60°C for 8 hours; after the reaction, remove the solvent under reduced pressure, add 10 mL of ethyl acetate to the residue, wash twice with 20 mL of saturated brine; wash the organic layer with anhydrous Na 2 SO 4 After drying and concentration under reduced pressure, it was separated and purified by column chromatography (eluent: ethyl acetate / petroleum ether=1 / 8) to obtain 0.055 g of a colorless solid with a yield of 83.0%.
[0029]
[0030] viscous liquid. 1 H NMR (400MHz, CDCl 3 )δ:7.98-7.96(m,2H),7.93(dd,J H-H =7.8 Hz,J H-H =1.3Hz,1H),7.68-7.59(m,2H),7.47(t,J H-H =7.8Hz,2H),7.41-7.37(m,2H), 4.27(t,J H-H =7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com