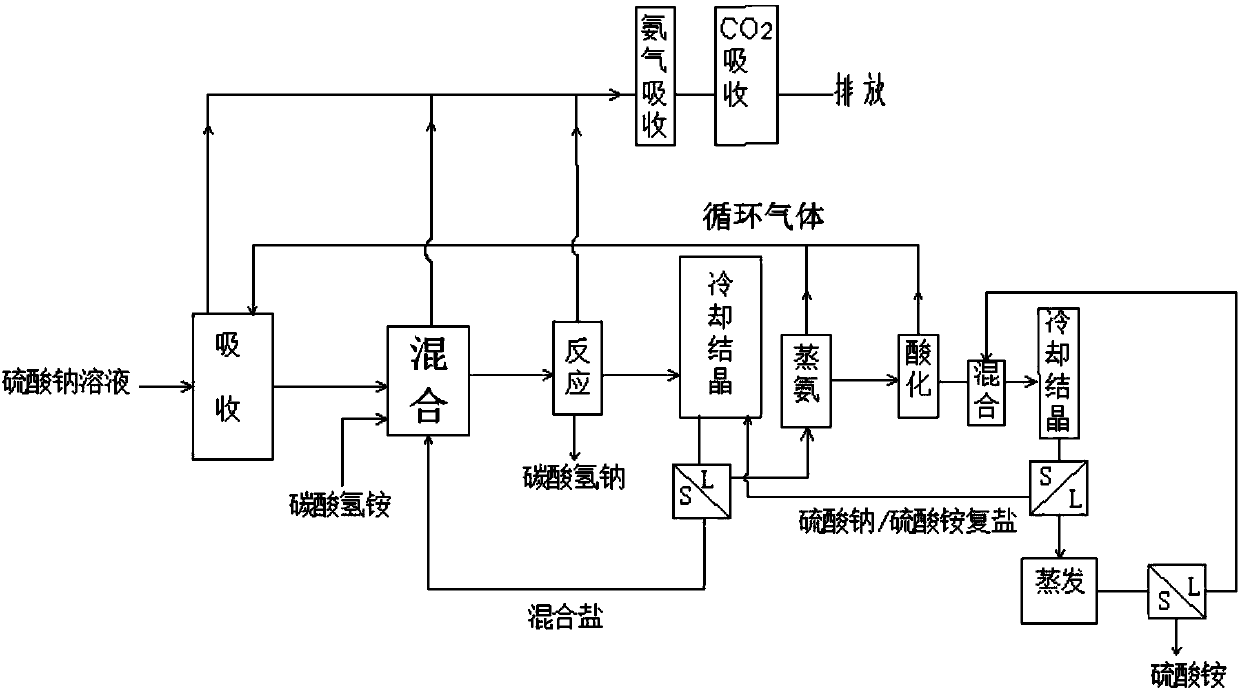
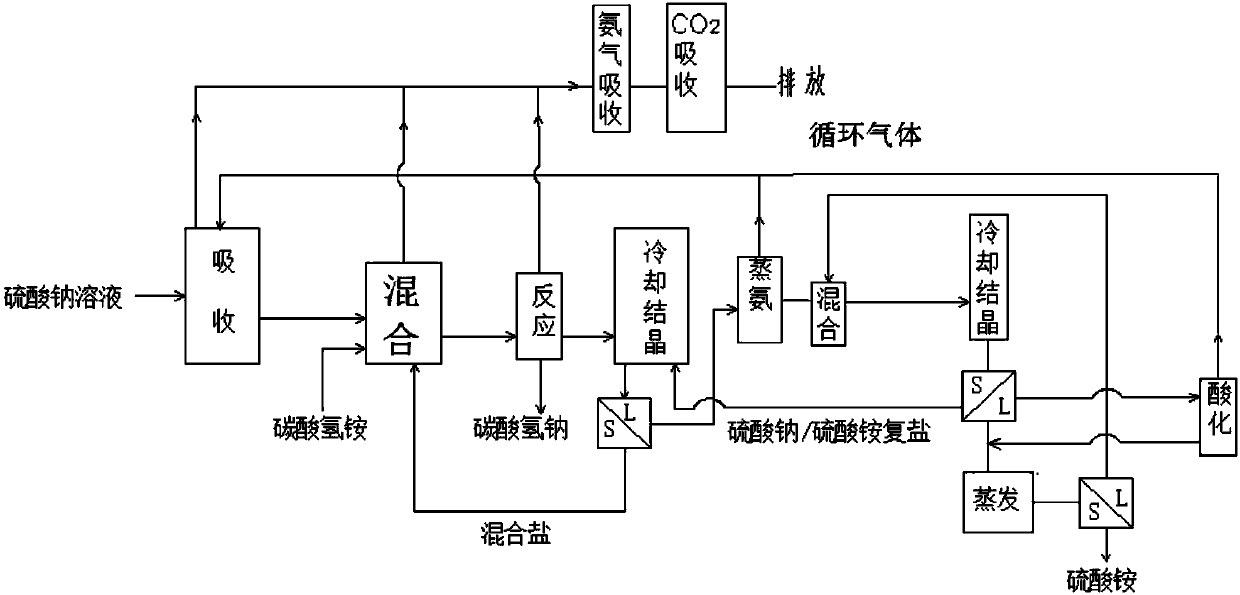
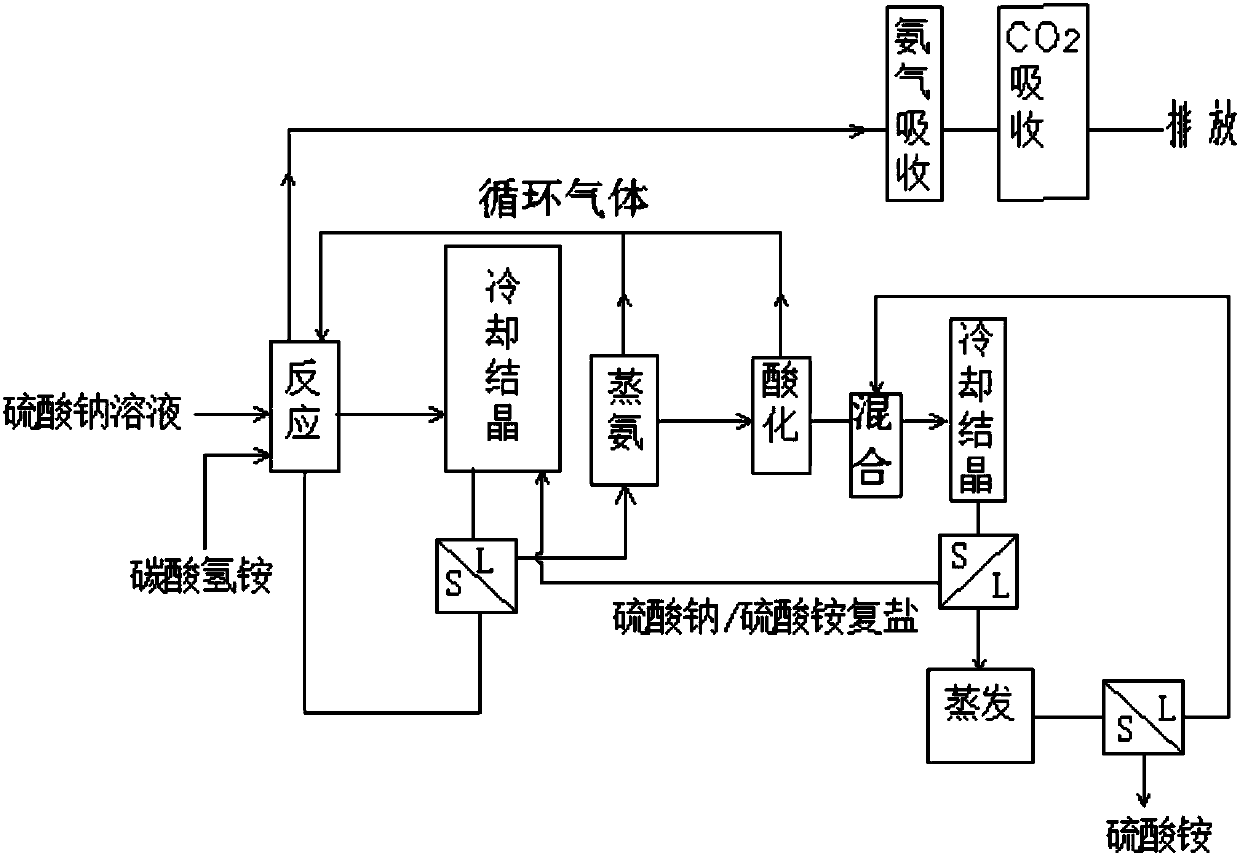
Method for producing sodium hydrogen carbonate and ammonium sulfate by using sodium sulfate solution
A technology of sodium sulfate solution and sodium bicarbonate, applied in chemical instruments and methods, carbonate preparations, ammonia compounds, etc., can solve the problems of low gas absorption utilization rate, lower raw material utilization rate, and difficulty in ammonia gas treatment, and achieve The effect of saving equipment, high utilization rate of raw materials, and low residual ammonia and carbon dioxide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Take 100kg of sodium sulfate solution obtained by sodium desulfurization, which contains 27.1% (1.91mol / L) of sodium sulfate, 2.37% of sodium bicarbonate, 0.4% of sodium chloride, and 70.14% of water. The solution is cooled at 0°C and sent to the absorption The tower is sprayed, and at the same time, 0.483kg of ammonia and 1.25kg of carbon dioxide obtained by distilling ammonia are sent to the bottom of the absorption tower. The obtained absorption liquid contained 25.26 kg of sodium sulfate.
[0074] Control the temperature in the mixer to 0°C, send the absorption liquid into the mixer, and add 28.1 kg of ammonium bicarbonate and frozen ammonium bicarbonate / sodium sulfate mixed salt to the mixer to obtain a mixed slurry, which is pumped into In the reactor, the temperature was raised to 38°C, the pressure was raised to 0.14MPa, and the reaction was carried out for 1.5 hours to obtain the heavy alkali and the reaction mother liquor. The composition of the obtained heavy...
Embodiment 2
[0083] Get sodium sulfate solution 110kg, wherein contain sodium sulfate 27.2%, sodium bicarbonate 2.47%, sodium chloride 0.3%, water 70.24%. After the solution is cooled at 0°C, it is sent to the absorption tower for spraying, and at the same time, the ammonia and carbon dioxide obtained from ammonia distillation are sent to the bottom of the absorption tower. The obtained absorption liquid contained 25.35 kg of sodium sulfate.
[0084] Control the temperature in the mixer to 0°C, send the absorption liquid into the mixer, and add 27.89 kg of ammonium bicarbonate to the mixer and cool the resulting ammonium bicarbonate / sodium sulfate mixed salt to obtain a mixed slurry, and send the mixed slurry into In the reactor, raise the temperature to 37°C, raise the pressure to 0.14MPa, and react for 1.5 hours to obtain heavy alkali and reaction mother liquor. The composition of the obtained heavy alkali is shown in Table 6.
[0085] Table 6 heavy alkali composition
[0086] ...
Embodiment 3
[0092] Get lithium carbonate to produce sodium sulfate by-product 100kg, and its composition is as shown in table 8. Use 57.6kg of water to absorb the ammonia and carbon dioxide obtained by distilling ammonia at 5°C; control the temperature in the mixer to 5°C, send the absorption liquid into the mixer and add 100kg of sodium sulfate by-product, 52.7kg of ammonium bicarbonate solid and sulfuric acid The mixed slurry of sodium / ammonium bicarbonate mixed salt was pumped into the reactor, and the pressure was increased to 0.14MPa at 38°C, and the sodium bicarbonate was separated after 2 hours of reaction. Sodium bicarbonate one-way output is 35.33kg, and its composition is as shown in table 9 below. The filtrate after the separation of sodium bicarbonate is carried out after the processing steps such as crystallization, evaporation, cooling identical with embodiment 1, isolate the ammonium sulfate product, and its composition is as shown in table 10:
[0093] Table 8 Lithium car...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com