Synthesis method of diclofenac sodium impurities B
A technology of diclofenac sodium and impurities, applied in the field of drug synthesis, can solve the problems of difficult raw materials, high cost, low yield, etc., and achieve the effect of cheap raw materials, short steps and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Synthesis of Example 1 Compound 2-[(2,6-dichlorophenyl)amino]toluene (intermediate 1)
[0035] Add 2,6-dichloroaniline (0.1mol), o-bromotoluene (0.1mol), potassium carbonate (0.3mol) and N,N-dimethylformamide (100ml) into a 250ml three-necked flask, stir and heat to 80°C, keep stirring for 4 hours, cool down to 20-25°C, pour the reaction system into 150ml of water, extract with ethyl acetate (200ml), separate, wash with water (150ml*2), dry over anhydrous sodium sulfate, and concentrate to obtain Crude product, directly vote for the next step.
Embodiment 2
[0036] Embodiment 2 compound 2-[(2,6-dichlorophenyl) amino] the synthesis of benzoic acid (intermediate 2)
[0037] Add the crude product obtained above, potassium permanganate (0.2mol) and 5% sodium hydroxide solution (200ml) into a 500ml three-neck flask, stir at room temperature for 6h, adjust the pH to 6 with dilute hydrochloric acid, and precipitate solids, filter and wash with water, After drying, 20.3 g of brown solid was obtained.
Embodiment 3
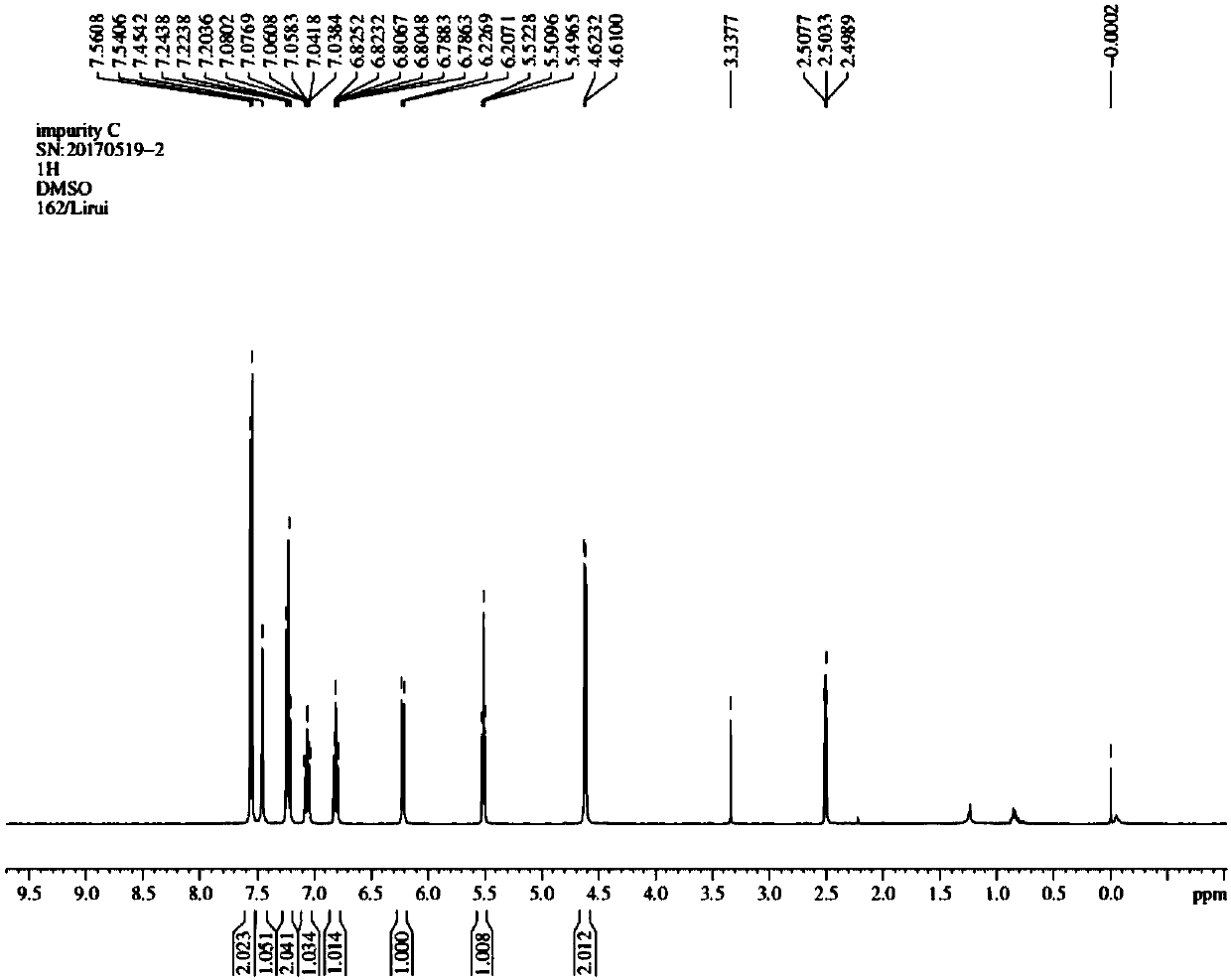
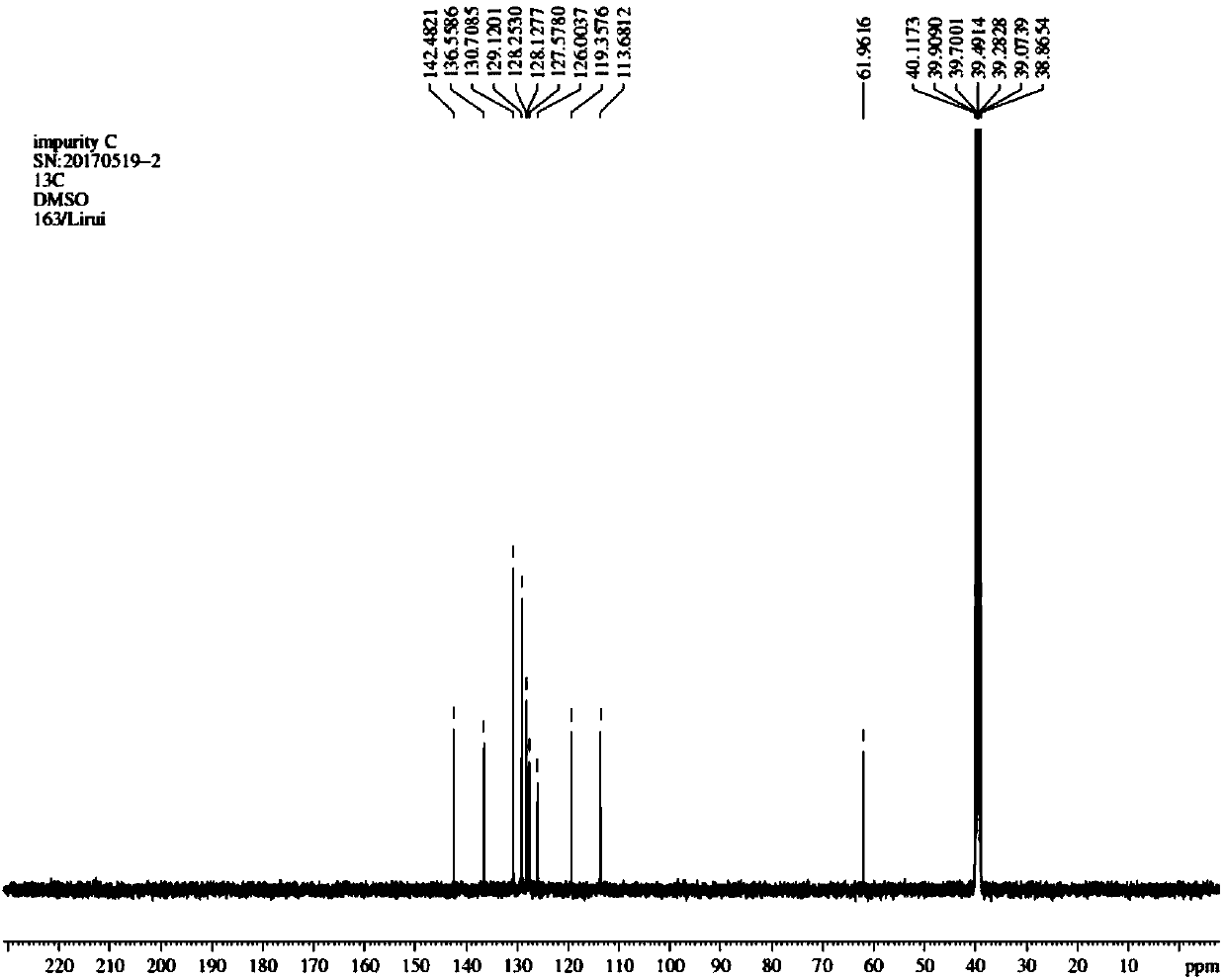
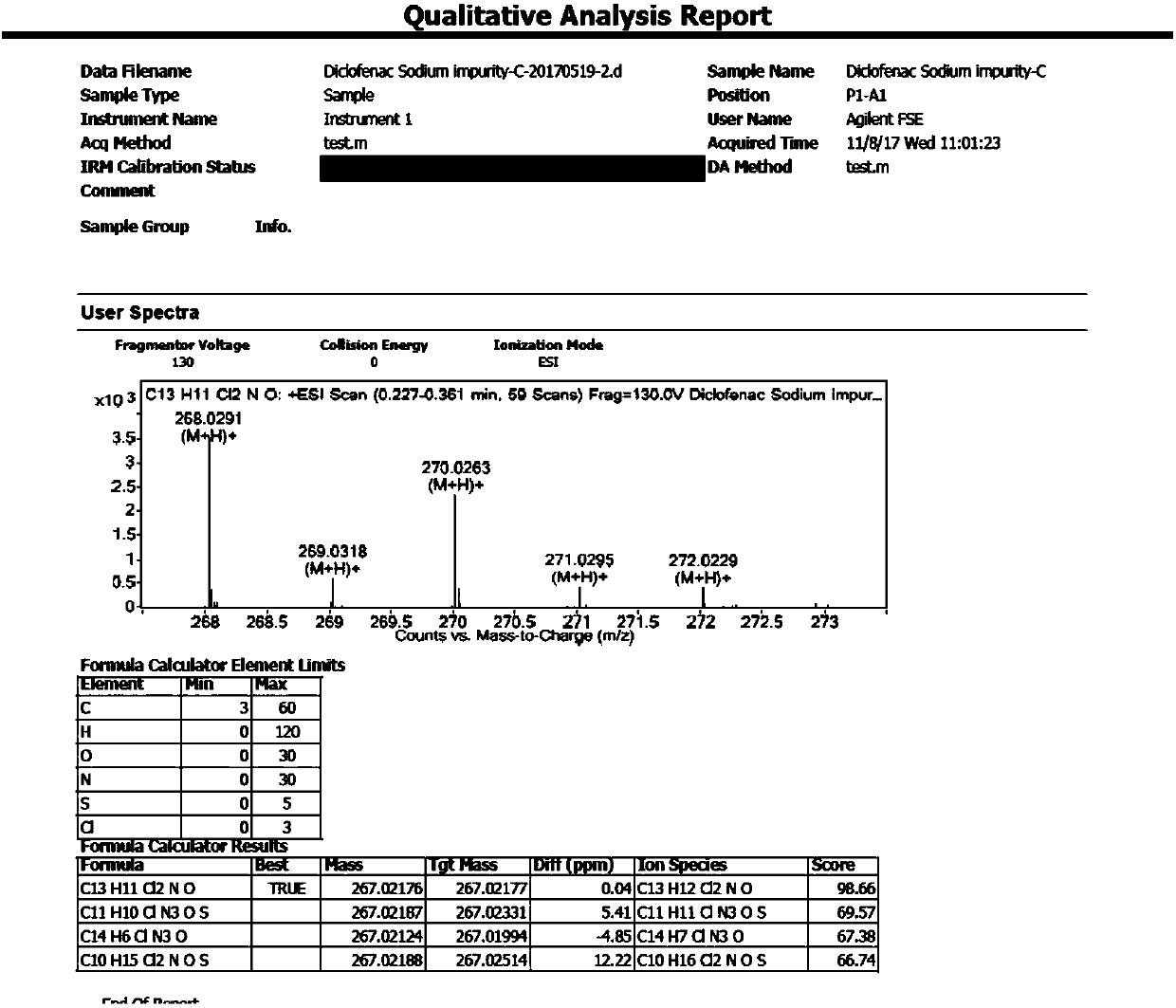
[0038] Synthesis of Example 3 Compound 2-[(2,6-dichlorophenyl)amino]benzyl alcohol (diclofenac sodium impurity C)
[0039] Add the above-prepared crude product (20.3g) and 100ml of tetrahydrofuran into a 250ml three-neck flask, stir, add 5.0g of lithium aluminum tetrahydride in batches, heat to 40°C, stir for 2h, cool down to 20°C, and pour the reaction system into 100ml of ice water, extracted with ethyl acetate (150ml*2), combined organic phases, washed with saturated sodium chloride solution (150ml), dried over anhydrous sodium sulfate, concentrated, column chromatography (petroleum ether: ethyl acetate = 20:1 ), to obtain 12.6g of white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com