Method for preparing arene by carrying out high-selectivity catalytic transfer hydrogenation on lignin derivative
A technology for catalytic transfer hydrogenation and lignin, applied in the directions of heterogeneous catalyst chemical elements, chemical instruments and methods, biological raw materials, etc., can solve the problem of difficult utilization of lignin pyrolysis products, and achieve enhanced ether bond cleavage and hydrogenation. Deoxidation performance, good deoxidation activity at low temperature, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
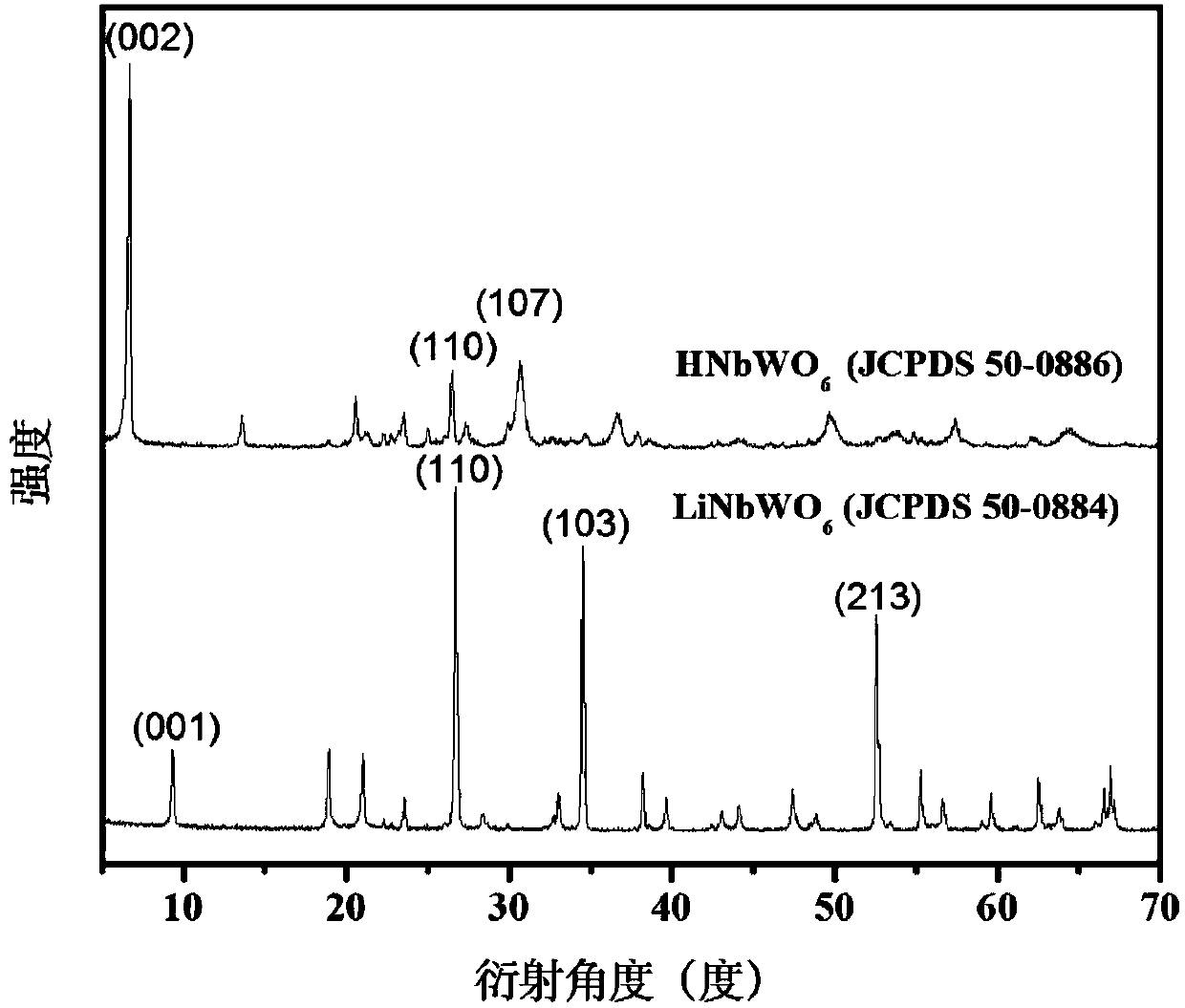
[0021] Example 1: 0.5wt%Pt / 20HNbWO 6 Preparation of / MWNCTs catalyst
[0022] The first choice is the treatment of supported carbon nanotubes. Weigh 5g of MWNCTs, add it into a round-bottomed flask filled with 250mL of concentrated nitric acid, ultrasonicate at 30°C for 3h, and collect the preliminarily treated carbon nanotubes by suction filtration. Add the preliminarily treated carbon nanotubes into the mixed acid solution of concentrated nitric acid and concentrated sulfuric acid with a volume ratio of 3:1, reflux overnight at 50°C, filter with suction, and wash repeatedly with a large amount of deionized water until the pH of the filtrate is neutral . After treatment, the carbon nanotubes were dried at 80°C for future use.
[0023] Preparation of LiNbWO by High Temperature Calcination 6 , the specific operation method is as follows: Weigh Li according to the stoichiometric coefficient 2 CO 3 , Nb 2 o 5 and WO 3 , after being mechanically mixed in a ball mill and c...
Embodiment 2
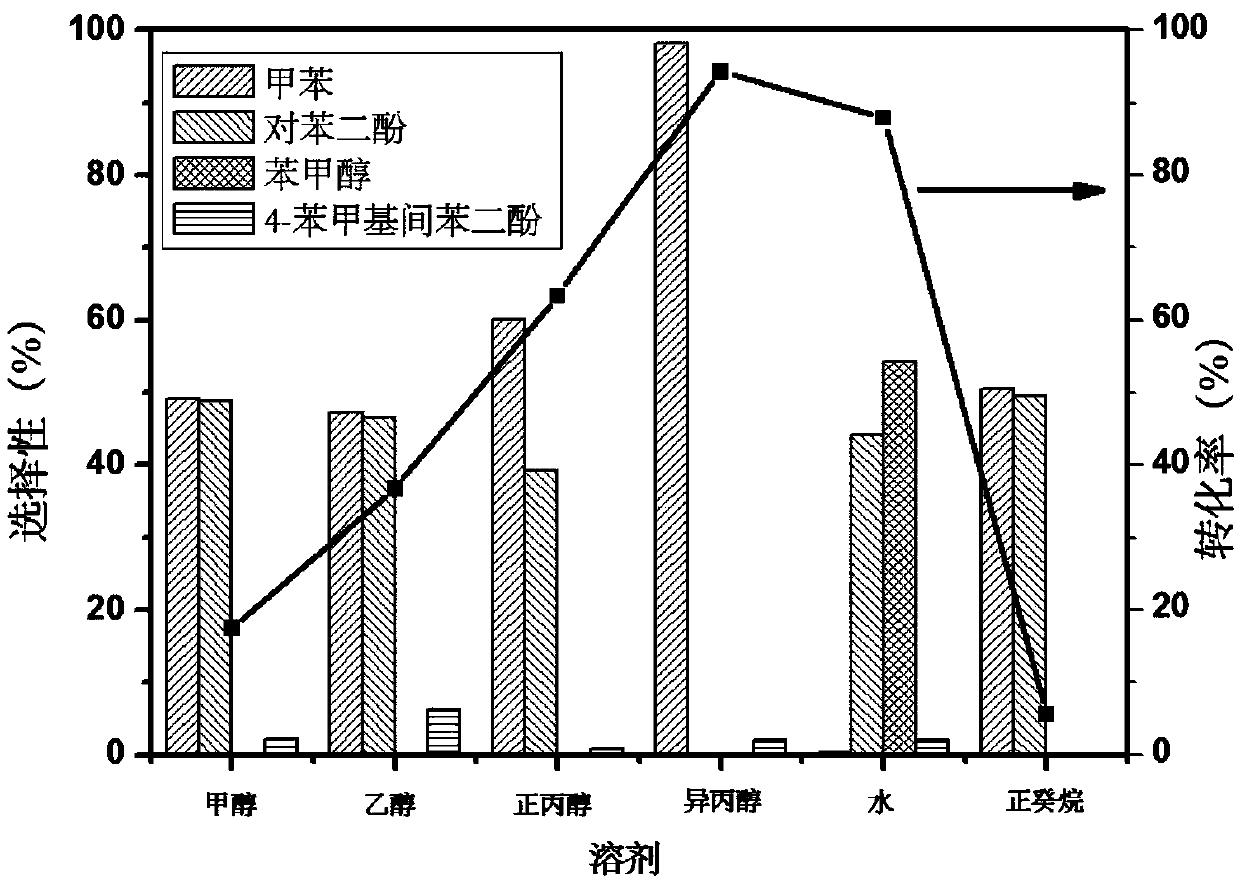
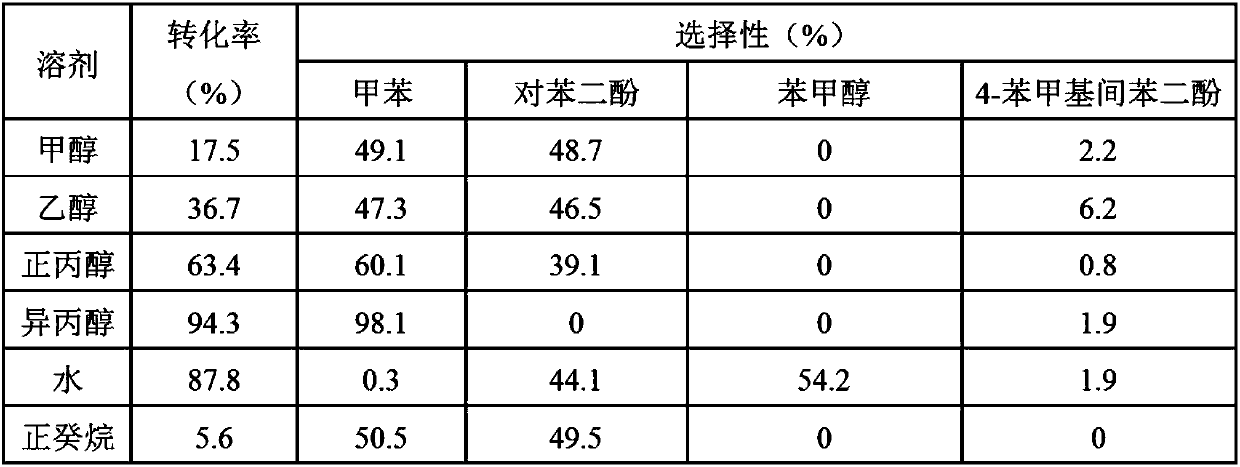
[0026] Embodiment 2: 4-benzyloxyphenol hydrodeoxygenation reaction (influence of solvent) in autoclave
[0027] In a typical 4-benzyloxyphenol deoxygenation experiment, the amount of catalyst used was 0.05 g, the solvent was 20 mL (methanol, ethanol, n-propanol, isopropanol, water, n-decane), and the internal standard n-dodecane was 0.3 g, 0.5 g of the substrate 4-benzyloxyphenol. Before the reaction, nitrogen was purged 3 times, and the timer was started when the temperature reached the target temperature. Reaction conditions: rotation speed 700rpm, temperature 240°C, reaction time 3h. The product was qualitatively analyzed using Agilent 5977A MSD and 7890B GC, and the substrate conversion rate and product selectivity were quantitatively analyzed using A90GC.
[0028] The result of the reaction is as follows:
[0029]
Embodiment 3
[0030] Embodiment 3: 4-benzyloxyphenol hydrodeoxygenation reaction (influence of temperature) in autoclave
[0031] In the experiment of 4-benzyloxyphenol deoxygenation, 0.5wt%Pt / 20HNbWO 6 The catalyst dosage / MWCNTs is 0.05g, the solvent is 20mL isopropanol, the internal standard n-dodecane is 0.3g, and the substrate 4-benzyloxyphenol is 0.5g. Before the reaction, nitrogen was purged 3 times, and the timer was started when the temperature reached the target temperature. Reaction conditions: temperature 160-260°C, rotation speed 700rpm, reaction time 3h. The product was qualitatively analyzed using Agilent 5977A MSD and 7890B GC, and the substrate conversion rate and product selectivity were quantitatively analyzed using A90GC.
[0032]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com