Method for preparing C6-C8 aromatics with mixed aromatics containing polycyclic aromatic hydrocarbon and di-cyclic aromatic hydrocarbon
A technology for bicyclic aromatics and mixed aromatics, applied in the treatment of hydrocarbon oil, hydrocarbon oil treatment products, petroleum industry, etc., can solve the problems of oversaturation, reaction temperature unfavorable cracking reaction of aromatics, etc., to improve conversion efficiency, reduce equipment investment and operation Cost, the effect of efficient use of fossil resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of typical catalysts:
[0036] (1) Preparation of selective hydrogenation saturation catalyst Ni / SBA-15: Dissolve the weighed nickel nitrate hexahydrate in water, load it on the molecular sieve SBA-15 by equal volume impregnation method, then let it stand for 2h, at 60°C Dry at low temperature, repeat this process until the nickel nitrate aqueous solution is completely impregnated, and then dry at 120°C overnight to obtain Ni / SBA-15 impregnating agent. When in use, hydrogen reduction is first performed in the fixed bed of the selective hydrogenation saturation section, and the reduction condition is 500°C for 2 hours.
[0037] (2) Preparation of hydrocracking catalyst Pt / Y: Load the weighed aqueous solution of chloroplatinic acid onto the Y-type molecular sieve by equal volume impregnation method, then let it stand for 2 hours, and dry it at 60 ° C. Repeat this process Process until the chloroplatinic acid aqueous solution is completely impregnated, and then ...
Embodiment 2
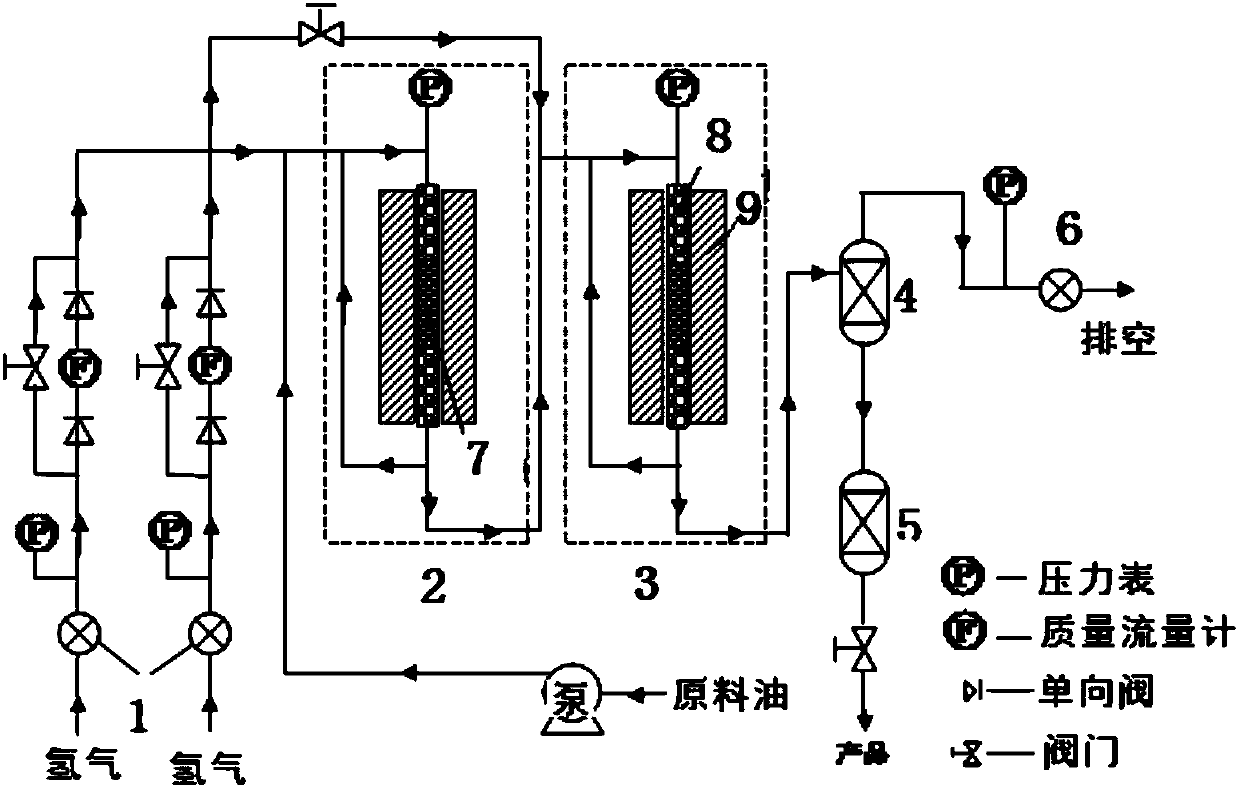
[0039] Catalytic conversion experiment: The reaction is carried out in a reaction device composed of two fixed beds in series, or only in a fixed bed reactor in the hydrocracking section according to the raw material composition.
[0040] (1) Filling of selective hydrogenation saturated catalyst: Add a certain amount of selective hydrogenation saturated catalyst into the fixed bed reactor, and fill a certain amount of ceramic balls at both ends, so that the center of the catalyst is at the best hot spot of the fixed bed.
[0041] (2) loading of the hydrocracking catalyst: when the catalyst is a metal-acidic bifunctional catalyst, the loading method is described in (1) in Example 2; when the catalyst is a mixed catalyst system composed of a hydrogenation catalyst and a molecular sieve acid catalyst , first mix the two catalysts, and then fill them in the manner described in (1) in Example 2.
[0042] Typical reaction conditions: After the catalyst has been reduced and cooled, t...
Embodiment 3
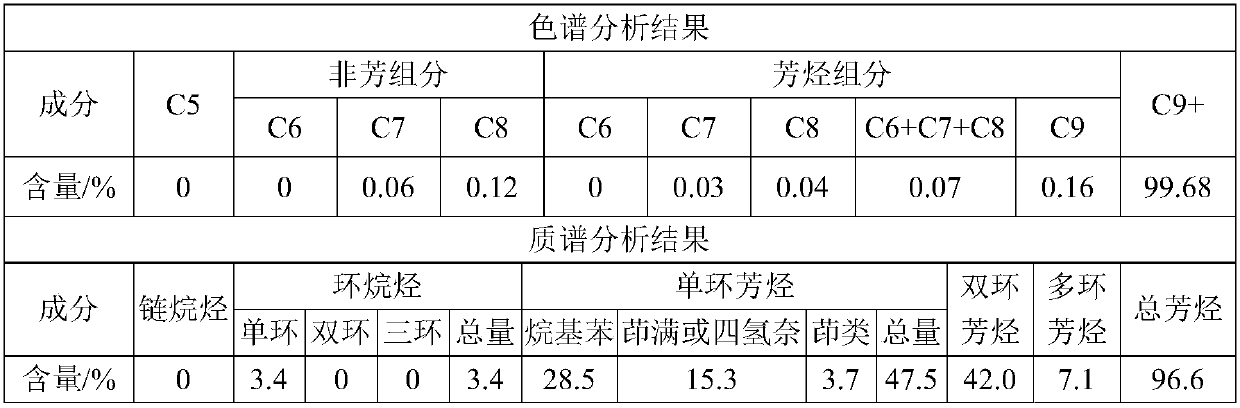
[0049] The mixed aromatics raw material containing polycyclic aromatic hydrocarbons is a reformed heavy aromatics, and its qualitative analysis results by chromatography and mass spectrometry are shown in Table 1.
[0050] Table 1. Chromatographic and mass spectrometric analysis results of certain reformed heavy aromatics
[0051]
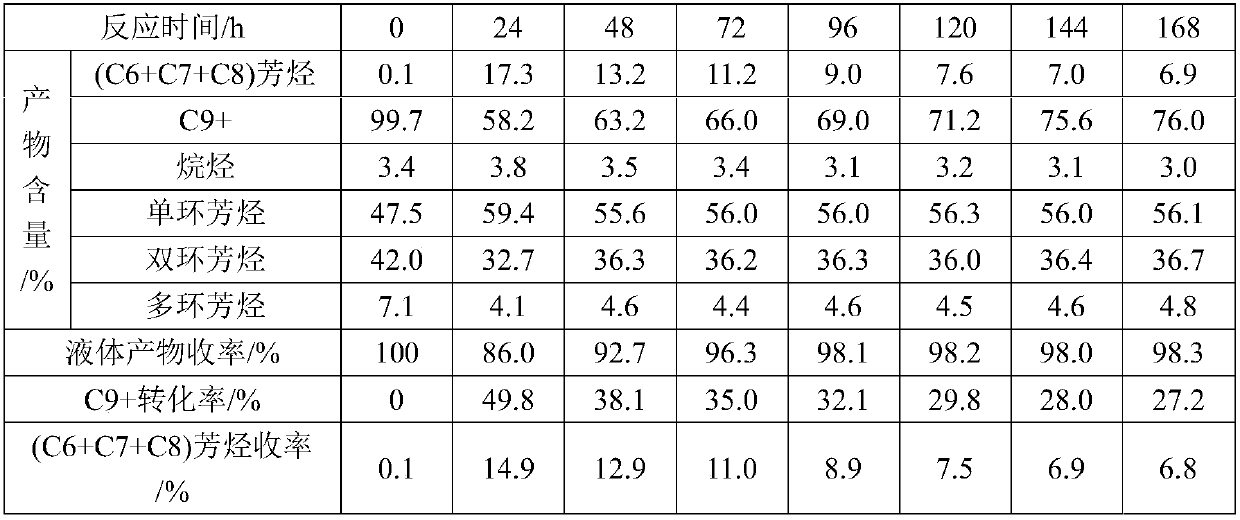
[0052] As can be seen from the results in Table 1, C in the raw material 6 ~C 8 The total content of aromatics is only 0.07%, and the C element is mainly C 9 + component present, C 9 + The content is as high as 99.68%. Mass spectrometry analysis shows that the raw material is mainly composed of aromatics, with a content as high as 96.6%, of which the contents of monocyclic and bicyclic aromatics are 47.5% and 42.0%, respectively, and the content of polycyclic aromatics is 7.1%. The high aromatic content of raw oil has certain advantages for the preparation of BTX.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com