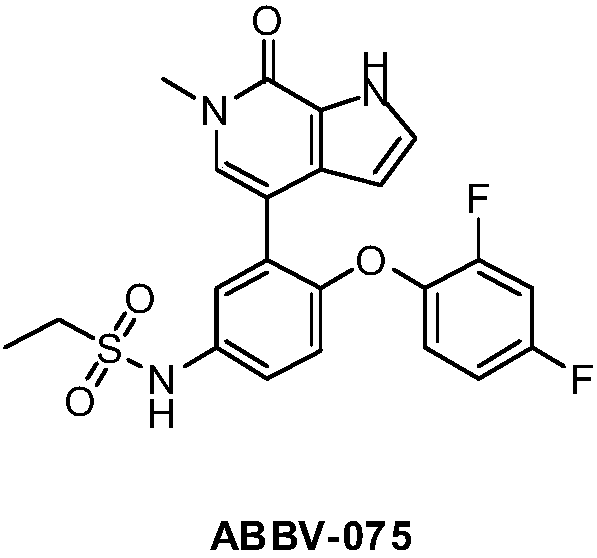
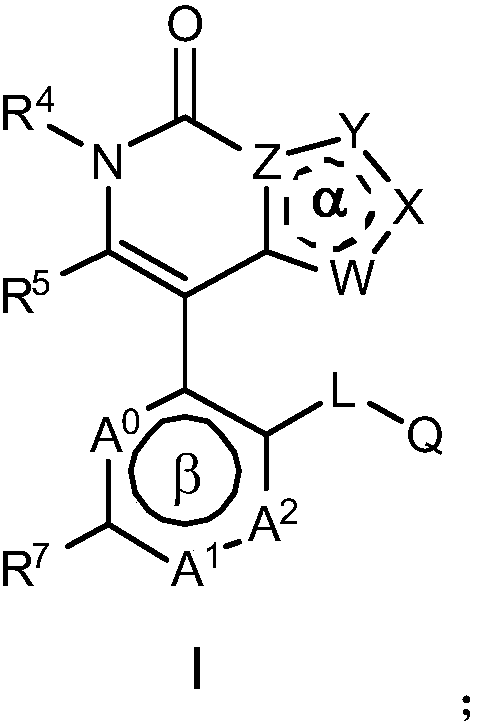
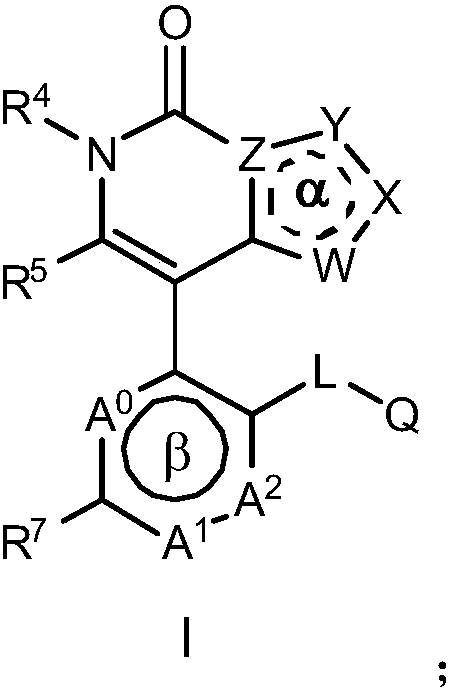
Nitrogen-containing heterocyclic ring compounds, and preparation method, pharmaceutical compositions and application thereof
A nitrogen heterocycle and compound technology, applied in the field of nitrogen-containing heterocycle compounds, can solve the problems of weak binding between bromodomain and acetylated protein, influence of druggability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0368] Example 1: N-(4-(2,4-difluorophenoxy)-3-(5-methyl-4-oxo-4,5-dihydro-1H-pyrrolo[3,2- c] pyridin-7-yl) phenyl) ethanesulfonamide (I-1)
[0369]
[0370] Step 1: 2-Bromo-1-(2,4-difluorophenoxy)-4-nitrobenzene
[0371]
[0372] 2-Bromo-1-fluoro-4-nitrobenzene (6.60g, 30.00mmol), 2,4-difluorophenol (3.90g, 30.00mmol) and cesium carbonate (19.50g, 60.00mmol) were dissolved in dimethyl sulfoxide (70 mL) and stirred at 110°C for 2 hours. The reaction solution was poured into water (100 mL), extracted with ethyl acetate (60*3 mL). The organic phase was washed with water and brine, dried (anhydrous sodium sulfate), filtered and concentrated to give the crude title compound (9.90 g, 100%), which was directly used in the next reaction.
[0373] Step 2: 3-Bromo-4-(2,4-difluorophenoxy)aniline
[0374]
[0375] Compound 1A (9.9g, 30.00mmol), iron powder (8.40g, 150.00mmol) and ammonium chloride (3.21g, 60.00mmol) were dissolved in a mixture of tetrahydrofuran / ethanol / wate...
Embodiment 2
[0409] Example 2: N-(4-(2,4-difluorophenoxy)-3-(5-methyl-4-oxo-4,5-dihydro-1H-pyrrolo[3,2- c] pyridin-7-yl) phenyl) -N-methylethanesulfonamide (I-2)
[0410]
[0411] Step 1: N-(4-(2,4-difluorophenoxy)-3-(5-methyl-4-oxo-1-((2-(trimethylsilyl)ethoxy) Methyl)-4,5-dihydro-1H-pyrrolo[3,2-c]pyridin-7-yl)phenyl)-N-methylethanesulfonamide
[0412]
[0413] Compound 1L (100mg, 0.17mmol) was dissolved in N,N-dimethylformamide (2mL), slowly added dropwise to sodium hydride (60% oil dispersion, 14mg, 0.34 mmol) in N,N-dimethylformamide (2 mL) solution. The reaction solution was stirred at 0°C for 30 minutes, then methyl iodide (37 mg, 0.26 mmol) was added, and the mixture was raised to room temperature and stirred for 2 hours. Water was added to the reaction system and extracted with ethyl acetate, the ethyl acetate layer was separated, washed with water and saturated brine, dried (anhydrous sodium sulfate), filtered and concentrated in vacuo to give the title compound (120mg, 6...
Embodiment 3
[0417] Example 3: N-(4-(2,4-difluorophenoxy)-3-(1,5-dimethyl-4-oxo-4,5-dihydro-1H-pyrrolo[3 ,2-c]pyridin-7-yl)phenyl)ethanesulfonamide (I-3)
[0418]
[0419] Step 1: 7-Bromo-4-methoxy-1-methyl-1H-pyrrolo[3,2-c]pyridine
[0420]
[0421] Compound 1G (400mg, 1.76mmol) was dissolved in tetrahydrofuran (4mL), and slowly added dropwise to a solution of sodium hydride (60% oil dispersion, 140mg, 3.52mmol) in tetrahydrofuran (4mL) at 0°C under nitrogen protection middle. The reaction solution was stirred at 0°C for 30 minutes, then iodomethane (374 mg, 2.64 mmol) was added, and stirring was continued at 0°C for 2 hours. Water was added to the reaction system and extracted with ethyl acetate, the ethyl acetate layer was separated, washed with water and saturated brine, dried (anhydrous sodium sulfate), filtered and concentrated in vacuo, the residue was separated and purified by flash chromatography (petroleum ether / ethyl acetate=10 / 1), the title compound (480 mg, 91%) was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com