N-phosphopeptide and protein enrichment material and preparation and application thereof
A technology for phosphorylation of peptides and proteins, applied in peptide preparation methods, analytical materials, chemical instruments and methods, etc., can solve the problems of phosphorylation modification loss, etc., and achieve the advantages of reduced loss, good enrichment effect and fast mass transfer Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
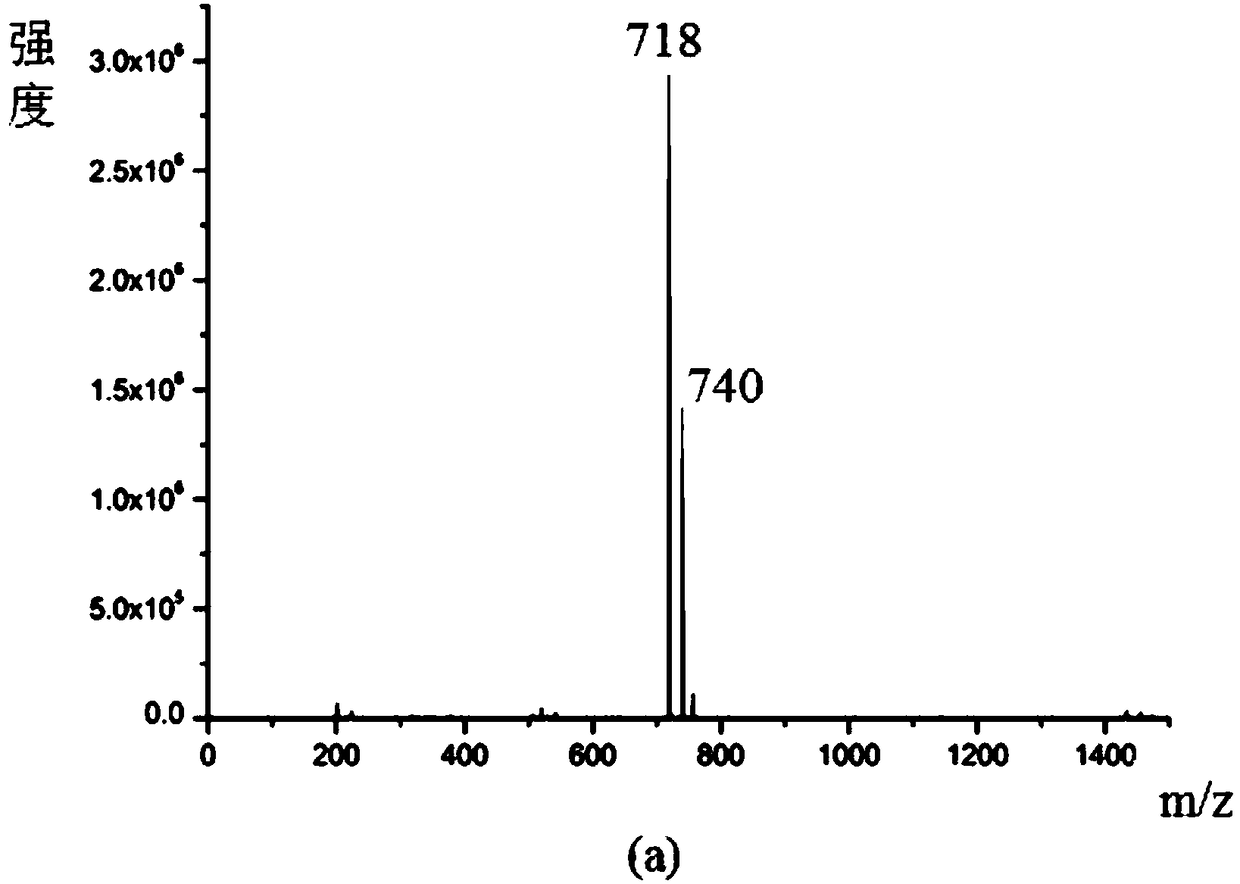
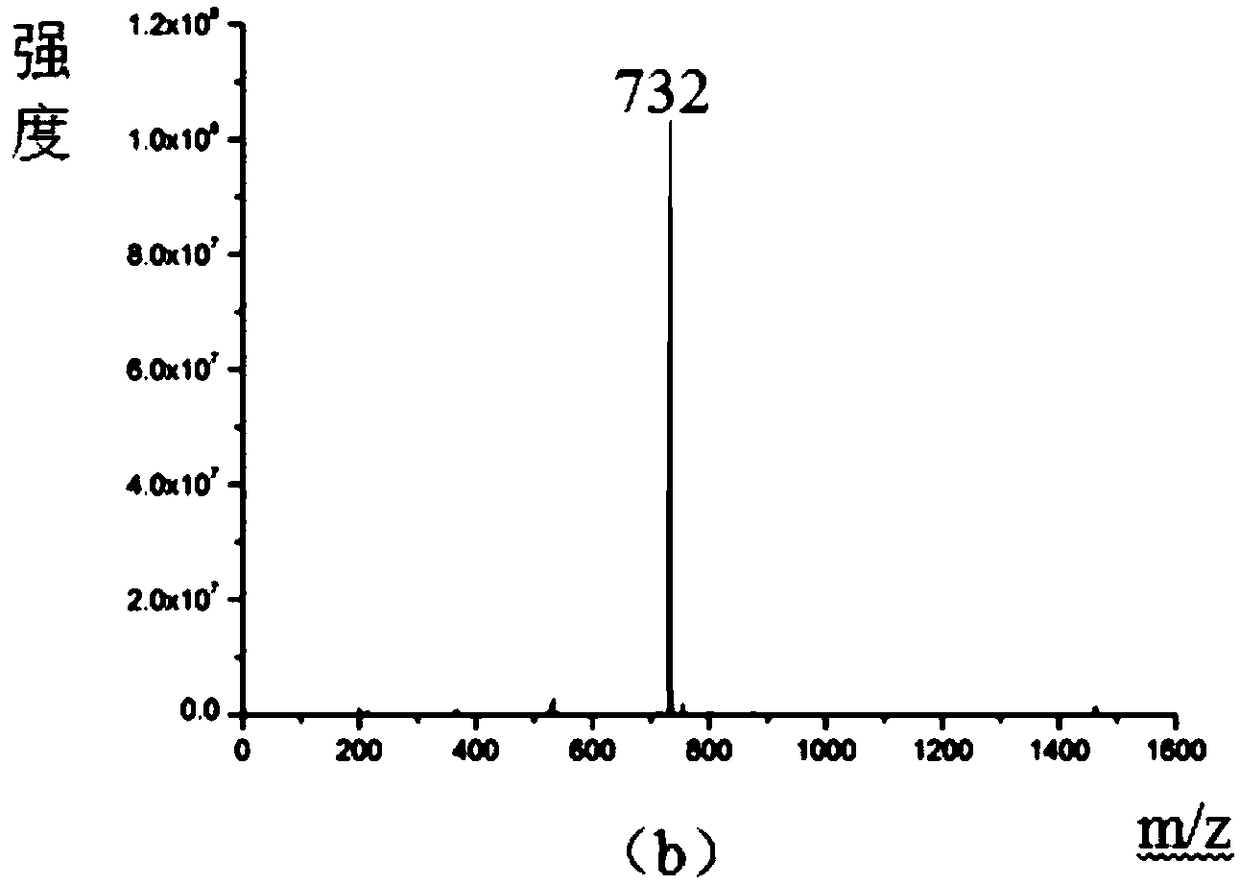
Image
Examples
Embodiment 1
[0046] 1. Preparation of sub-two-micron core-shell silica gel with vertical pores:
[0047] a) Mix the hydrolysate (6.7mL ammonia water, 5.1mL water and 70mL absolute ethanol) and 4mL ethyl orthosilicate, stir in a 22℃ water bath for 40min, and heat up to 55℃;
[0048] b) Add 0.64mL water and 4mL ethyl orthosilicate, react at 55°C for 40min;
[0049] c) Repeat step (2) 4 times to get Sead1;
[0050] d) Replace the Sead1 solution with an equal amount of newly prepared hydrolysate, and repeat step (2) 10 times to obtain Sead2.
[0051] e) Disperse Sead2 in 100mL water, add 1g cetyltrimethylammonium chloride, 5.8mL tridecane, 0.1-100mg ammonium fluoride and 0.1-10mL ammonia, react at 90°C for 24h to obtain NPs;
[0052] f) Sintering the dried NPs at 550°C for 6 hours to form the initial shell layer of core-shell microspheres.
[0053] g) Disperse NPs in 30 mL of 5N HCl solution, and reflux for 12 hours at 120°C.
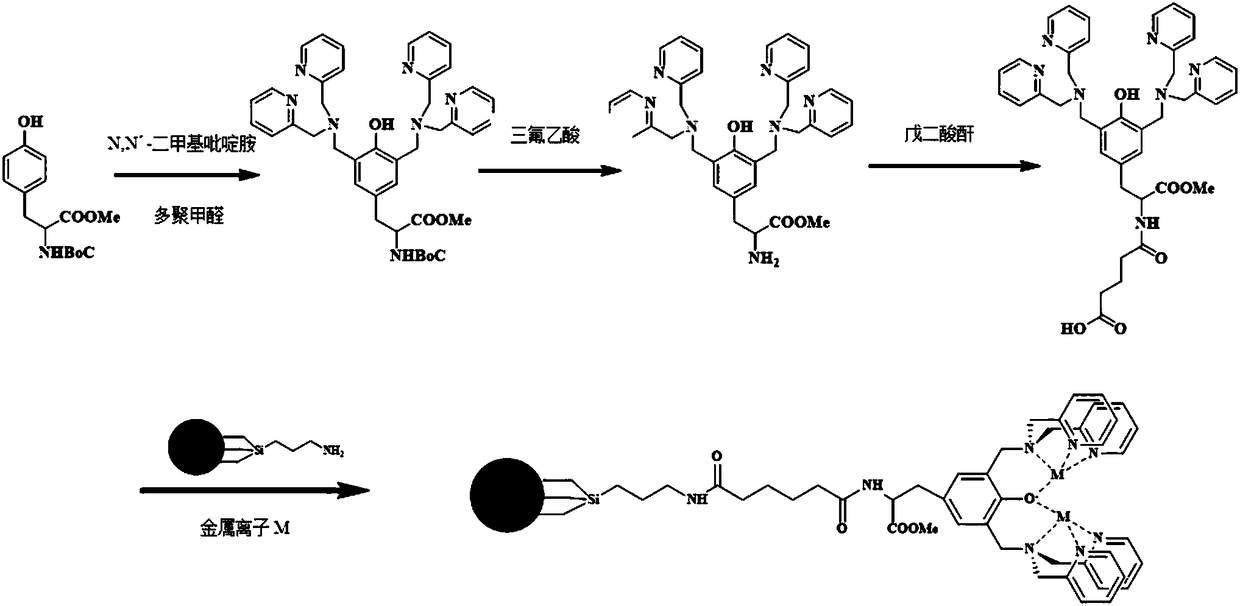
[0054] 2. Synthesis of a phosphate recognition functional molecule:
[0055] a) ...
Embodiment 2
[0065] 1. Preparation of sub-two-micron core-shell silica gel with vertical pores:
[0066] a) Mix the hydrolysate (6.7mL ammonia water, 5.1mL water and 70mL absolute ethanol) and 4mL ethyl orthosilicate, stir in a 22℃ water bath for 40min, and heat up to 55℃;
[0067] b) Add 0.64mL water and 4mL ethyl orthosilicate, react at 55°C for 40min;
[0068] c) Repeat step (2) 4 times to get Sead1;
[0069] d) Replace the Sead1 solution with an equal amount of newly prepared hydrolysate, and repeat step (2) 10 times to obtain Sead2.
[0070] e) Disperse Sead2 in 100mL water, add 1g cetyltrimethylammonium bromide, 5.8mL tridecane, 0.1-100mg ammonium fluoride and 0.1-10mL ammonia, react at 90°C for 24h to obtain NPs;
[0071] f) Sintering the dried NPs at 550°C for 6 hours to form the initial shell layer of core-shell microspheres.
[0072] g) Disperse NPs in 30mL 5N HCl solution, and reflux for 12h at 120°C.
[0073] 2. Synthesis of a phosphate recognition functional molecule:
[0074] a) 3.37g N,N'...
Embodiment 3
[0085] 1. Preparation of sub-two-micron core-shell silica gel with vertical pores:
[0086] a) Mix the hydrolysate (6.7mL ammonia, 5.1mL water and 70mL absolute ethanol) and 4mL ethyl orthosilicate, stir in a water bath at 22°C for 40min, and heat up to 55°C;
[0087] b) Add 0.64mL water and 4mL ethyl orthosilicate, react at 55°C for 40min;
[0088] c) Repeat step (2) 4 times to get Sead1;
[0089] d) Replace the solution of Sead1 with an equal amount of newly prepared hydrolysate, and repeat step (2) 10 times to obtain Sead2.
[0090] e) Disperse Sead2 in 100mL water, add 1g cetyltrimethylammonium bromide, 5.8mL tridecane, 0.1-100mg ammonium fluoride and 0.1-10mL ammonia, react at 90°C for 24h to obtain NPs;
[0091] f) Sintering the dried NPs at 550°C for 6 hours to form the initial shell layer of core-shell microspheres.
[0092] g) Disperse NPs in 30mL 5N HCl solution, and reflux for 12h at 120°C.
[0093] 2. Synthesis of a phosphate recognition functional molecule:
[0094] a) 3.37g N,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com