Preparation method of hexaaluminate catalysts with high specific surface areas
A high specific surface area, hexaaluminate technology, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem of specific surface area loss and achieve crystal grain The effect of small size, easy scale production, and high specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1.629g of La 2 o 3 Dissolve completely in excess nitric acid solution to produce La(NO 3 ) 3 solution, 3.579g of Mn(NO 3 ) 2 solution (mass fraction is 50%) and 41.681g of Al(NO3 ) 3 9H 2 O is sequentially added to La(NO 3 ) 3 In the solution, after the nitrate was completely dissolved, 66.749g of sucrose was added, and the solution was continuously stirred under heating at 100°C until the solution evaporated and concentrated to a yellow viscous sol. Afterwards, the obtained sol was dried in an oven at 120° C. for 12 hours, and the sol foamed and expanded to become a fluffy brown precursor. After grinding the precursor into powder, first raise the temperature to 1100°C at 3°C / min in an argon atmosphere and keep it warm for 2 hours, then switch the argon gas to air and continue to keep warm at 1100°C for 5 hours, cool down and pulverize by ball milling Get LaMnAl 11 o 19-δ Hexaaluminate catalyst.
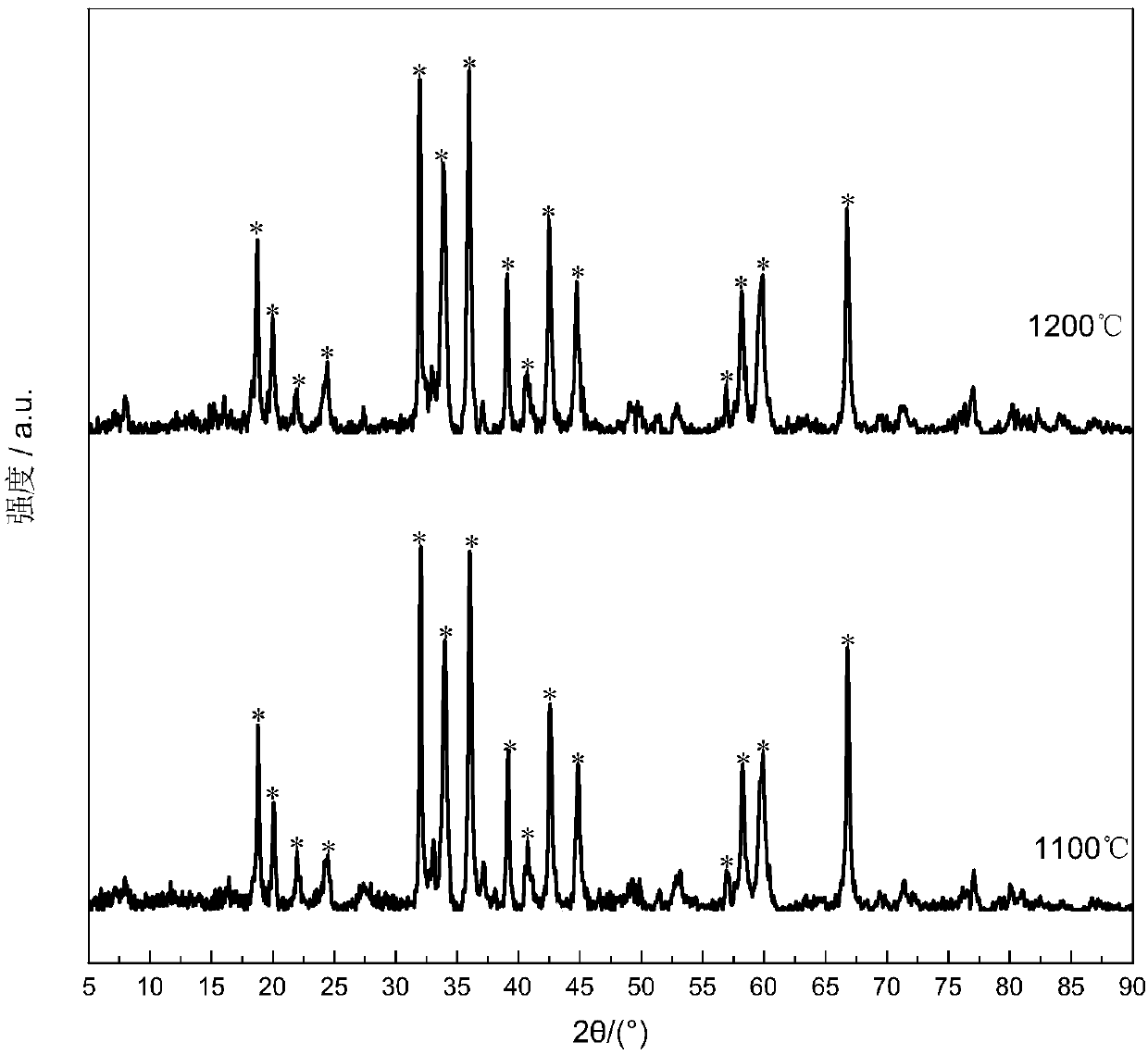
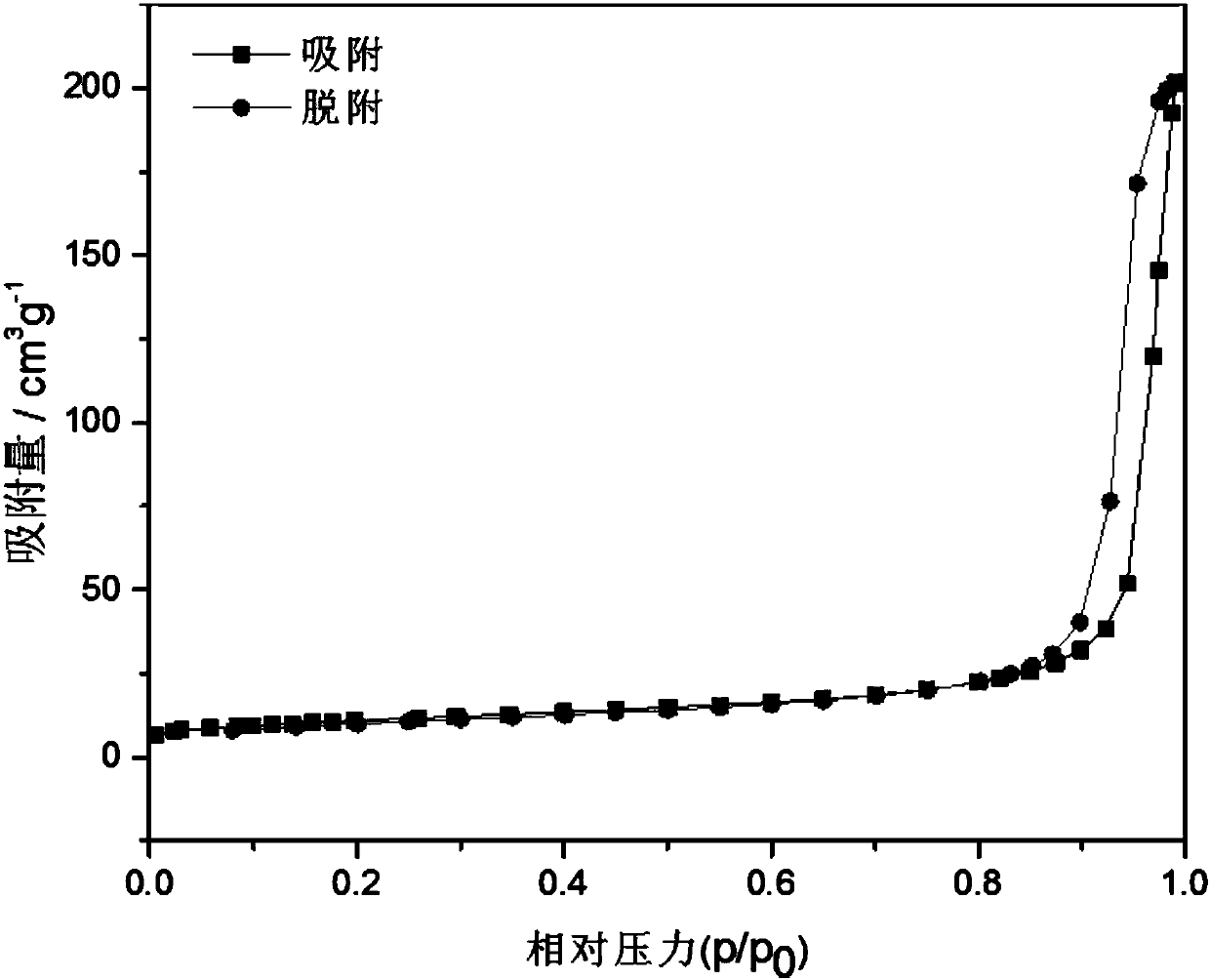
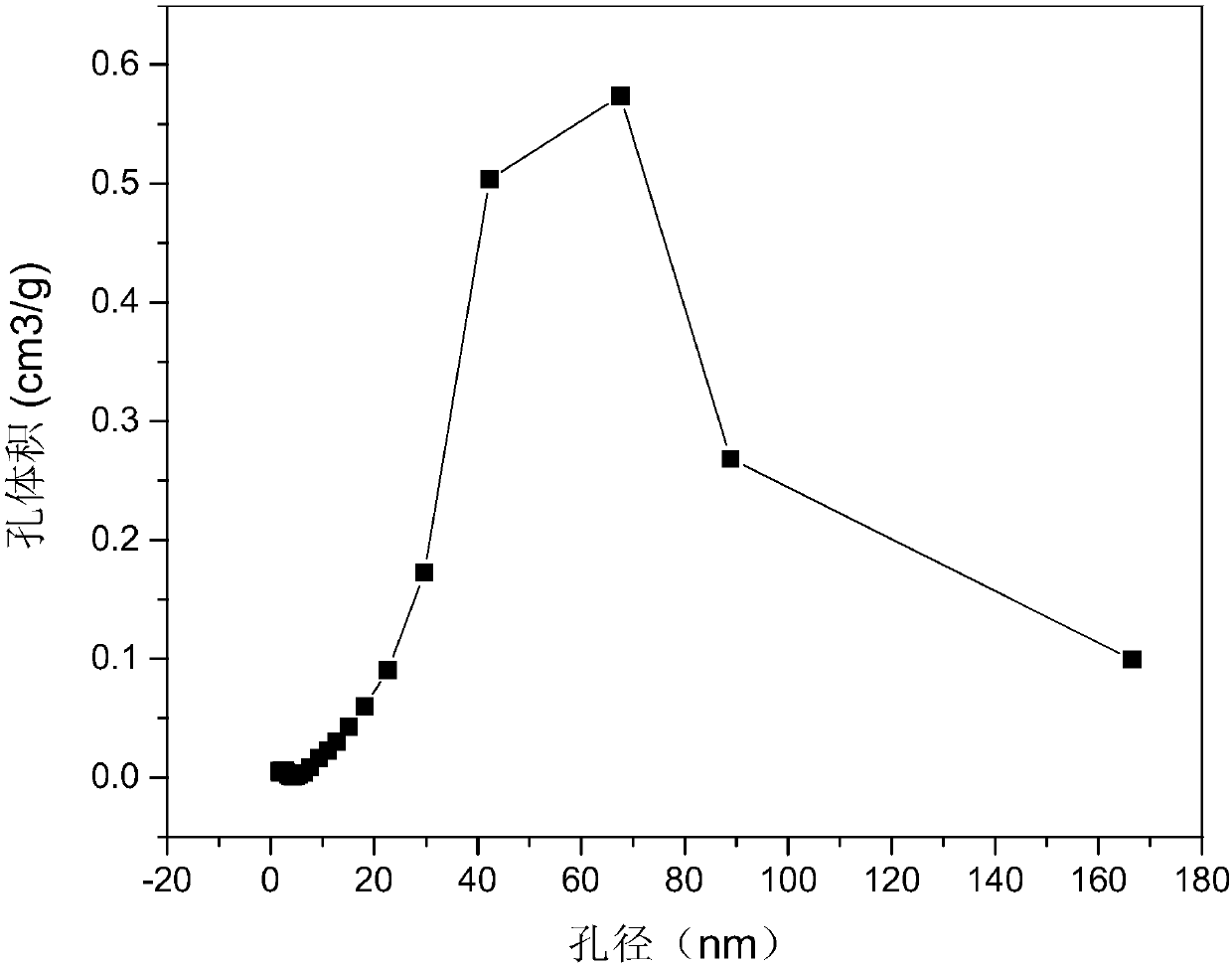
[0031] determined, as Figure 1 to Figure 4 As shown, the ob...
Embodiment 2
[0033] The difference from Example 1 is that the temperature of roasting and calcination is 1200°C, and the rest are the same. determined, as figure 1 Shown, the position of the characteristic peak of the XRD spectrogram of catalyst is the same as embodiment 1, does not exist such as Al 2 o 3 Miscellaneous phases were obtained with a single hexaaluminate compared to a surface area of 42m 2 / g LaMnAl 11 o 19-δ Hexaaluminate catalyst.
Embodiment 2
[0035] The difference with Example 1 is that, according to the general formula AAl 12-x B x o 19 Among them, x=0 is used for batching, and the rest are the same. After determination, the obtained LaAl 12 o 19 The characteristic peaks of the XRD spectrum of the hexaaluminate catalyst are compared with the standard XRD characteristic peaks of the hexaaluminate phase, which does not exist such as Al 2 o 3 Miscellaneous phases, with a single hexaaluminate phase, additionally, a specific surface area of 49m 2 / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com